Abstract.
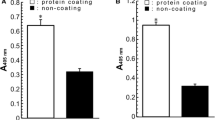
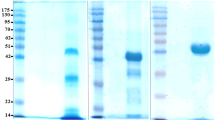
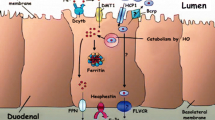
Lactoferrin (Lf), a prominent protein in milk, many other secretory fluids and white blood cells, is a monomeric, 80-kDa glycoprotein, with a single polypeptide chain of about 690 amino acid residues. Amino acid sequence relationships place it in the wider transferrin family. Crystallographic analyses of human Lf, and of the Lfs from cow, horse, buffalo and camel, reveal a highly conserved three-dimensional structure, but with differences in detail between species. The molecule is folded into homologous N- and C-terminal lobes, each comprising two domains that enclose a conserved iron binding site. Iron binding and release is accompanied by domain movements that close or open the sites, and is influenced by cooperative interactions between the lobes. Patches of high positive charge on the surface contribute to other binding properties, but the attached glycan chains appear to have little impact on structure and function.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baker, E.N., Baker, H.M. Lactoferrin. Cell. Mol. Life Sci. 62, 2531 (2005). https://doi.org/10.1007/s00018-005-5368-9
Published:
DOI: https://doi.org/10.1007/s00018-005-5368-9