Abstract
Objective
Cysteinyl leukotrienes (CysLTs), a group of inflammatory lipid mediators, are found elevated in obese-asthmatic patients. Leukotriene D4 (LTD4), a representative CysLT, is implicated in promoting lung inflammation and remodelling in allergic asthma, but its role in non-allergic asthma, especially in obese-asthmatic patients, is not known. Here, using primary human small airway epithelial cells (SAECs) we have investigated the mechanism of LTD4-induced inflammation and remodelling and assessed high proneness of obese mice to develop asthma upon challenge with allergen ovalbumin (OVA).
Methods
Primary human small airway epithelial cells (SAECs) were stimulated with different concentrations of LTD4 for different time intervals and various inflammatory markers were measured through cytokine array, membrane-based ELISA and Western blotting. An air–liquid interface (ALI) model of SAECs was used to study the effects of LTD4-induced remodelling in SAECs using Western blotting, H&E staining and PAS staining. Further, OVA-based murine model was used to examine the propensity of high-fat diet (HFD)-fed obese mice to develop asthma symptoms by studying the infiltration of inflammatory cells (assessed by bronchioalveolar lavage (BAL) cytology) and airway remodelling (assessed by histopathology) upon allergen exposure.
Results
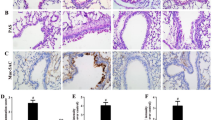
The human primary small airway epithelial cells (SAECs) treated with LTD4 showed significant alterations in the levels of inflammatory markers such as GM-CSF, TNF-α, IL-1β, EGF and eotaxin in dose- and time-dependent manner. Further, LTD4 enhanced the activation of inflammasomes as evidenced by increased levels of NALP3, cleaved caspase-1 and IL-1β. LTD4 also enhanced inflammation by increasing the expression of COX-2 in SAECs. The airway remodelling markers Vimentin and Muc5AC were found elevated in ALI culture of SAECs when stimulated with LTD4, as it also increased TGF-β levels and activation of Smad2/3 phosphorylation in SAECs. Last, sensitization and challenge of HFD-fed obese mice with OVA showed increased infiltration of inflammatory cells in BAL and enhanced levels of remodeling phenotypes like loss of cilia, mucus cell metaplasia and collagen deposition in mice lung tissues.
Conclusion
The results suggest that LTD4 could induce inflammatory response in human airway epithelial cell by activating NALP3 inflammasome. LTD4 could further promote airway epithelial cells’ remodelling through TGF-β/smad2/3-mediated pathway. Our in vivo results suggested that obesity predisposed the OVA challenged mice to develop lung inflammation and remodelling akin to asthma-like phenotypes during obesity.










Similar content being viewed by others
References
Salmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43(2):285–96.
Savari S, et al. Cysteinyl leukotrienes and their receptors: bridging inflammation and colorectal cancer. World J Gastroenterol. 2014;20(4):968–77.
Colazzo F, et al. Role of the cysteinyl leukotrienes in the pathogenesis and progression of cardiovascular diseases. Mediat Inflamm. 2017;2017:2432958.
Duah E, et al. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Sci Rep. 2013;3:3274.
Coffey MJ, Torretti B, Mancuso P. Adipokines and cysteinyl leukotrienes in the pathogenesis of asthma. J Allergy (Cairo). 2015;2015:157919.
Antczak A, et al. Exhaled eicosanoids and biomarkers of oxidative stress in exacerbation of chronic obstructive pulmonary disease. Arch Med Sci. 2012;8(2):277–85.
Yadav UC, Srivastava SK. Cysteinyl leukotrienes (CysLTs): role in obesity-induced asthma. Curr Mol Med. 2015;15(7):598–605.
Giouleka P, et al. Body mass index is associated with leukotriene inflammation in asthmatics. Eur J Clin Invest. 2011;41(1):30–8.
Yoshisue H, et al. Cysteinyl leukotrienes synergize with growth factors to induce proliferation of human bronchial fibroblasts. J Allergy Clin Immunol. 2007;119(1):132–40.
Ravasi S, et al. CysLT1 receptor-induced human airway smooth muscle cells proliferation requires ROS generation, EGF receptor transactivation and ERK1/2 phosphorylation. Respir Res. 2006;7:42.
Paruchuri S, et al. Leukotriene D(4) activates MAPK through a Ras-independent but PKCepsilon-dependent pathway in intestinal epithelial cells. J Cell Sci. 2002;115(Pt 9):1883–93.
Dholia N, Yadav UCS. Lipid mediator leukotriene D4-induces airway epithelial cells proliferation through EGFR/ERK1/2 pathway. Prostaglandins Other Lipid Mediat. 2018;136:55–63.
Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol. 2013;229(2):145–56.
Farkas AM, Kilgore TM, Lotze MT. Detecting DNA: getting and begetting cancer. Curr Opin Investig Drugs. 2007;8(12):981–6.
Bours MJ, et al. Adenosine 5ʹ-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404.
Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–21.
Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367(3):551–69.
Wang C. Obesity, inflammation, and lung injury (OILI): the good. Mediat Inflamm. 2014;2014:978463.
Singh N, et al. Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr Cancer. 2013;65(Suppl 1):36–43.
Sethi GS, Naura AS. Progressive increase in allergen concentration abrogates immune tolerance in ovalbumin-induced murine model of chronic asthma. Int Immunopharmacol. 2018;60:121–31. https://doi.org/10.1016/j.intimp.2018.04.047.
Martin-Garcia C, et al. Celecoxib, a highly selective COX-2 inhibitor, is safe in aspirin-induced asthma patients. J Investig Allergol Clin Immunol. 2003;13(1):20–5.
Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor beta and severe asthma: a perfect storm. Respir Med. 2014;108(10):1409–23.
Zhang M, et al. TGF-beta1 induces human bronchial epithelial cell-to-mesenchymal transition in vitro. Lung. 2009;187(3):187–94.
Dixon AE, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–35.
Centers for Disease Control and Prevention USA. Asthma and Obesity statistics for the US. 2017. https://www.cdc.gov/asthma/asthma_stats/asthma_obesity.htm.
McGovern T, et al. LTD(4) induces HB-EGF-dependent CXCL8 release through EGFR activation in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L808–15.
Profita M, et al. Cysteinyl leukotriene-1 receptor activation in a human bronchial epithelial cell line leads to signal transducer and activator of transcription 1-mediated eosinophil adhesion. J Pharmacol Exp Ther. 2008;325(3):1024–30.
Leikauf GD, et al. Cysteinyl leukotrienes enhance growth of human airway epithelial cells. Am J Physiol. 1990;259(4 Pt 1):L255–61.
Miller YI, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–48.
Liu W, et al. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm Res. 2014;63(1):33–43.
Pang L. COX-2 expression in asthmatic airways: the story so far. Thorax. 2001;56(5):335–6.
Kuwano T, et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. Faseb j. 2004;18(2):300–10.
Magazine R, Surendra VU, Chogtu B. Comparison of oral montelukast with oral ozagrel in acute asthma: a randomized, double-blind, placebo-controlled study. Lung India. 2018;35(1):16–20.
Wang X, et al. Micro-invasive embedding combined with montelukast sodium for children cough variant asthma:a randomized controlled trial. Zhongguo Zhen Jiu. 2017;37(3):259–64.
Lee HY, et al. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-beta/Smad signaling pathway in chronic asthma model. Allergy Asthma Immunol Res. 2017;9(1):25–34.
Acknowledgements
Neeraj Dholia acknowledges the receipt of Rajiv Gandhi National Fellowship from University Grant Commission (UGC), Government of India. Gurupreet S. Sethi acknowledges the UGC-BSR Senior Research Fellowship from UGC. Umesh C. S. Yadav thankfully acknowledges the award of Ramanujan Fellowship (SR/S2/RJN-102/2012) by Department of Science & Technology (DST)/Science and Engineering Research Board (SERB), Government of India. The Ramalingaswami Fellowship from Department of Biotechnology, Government of India (BT/RLF/Re-entry/36/2012) to Amarjit S. Naura is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dholia, N., Sethi, G.S., Naura, A.S. et al. Cysteinyl leukotriene D4 (LTD4) promotes airway epithelial cell inflammation and remodelling. Inflamm. Res. 70, 109–126 (2021). https://doi.org/10.1007/s00011-020-01416-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01416-z