Abstract
Objective and design
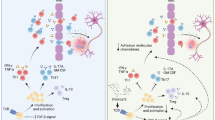
Multiple sclerosis (MS) is a debilitating autoimmune disease involving immune dysregulation of the pathogenic T helper 17 (Th17) versus protective T regulatory (Treg) cell subsets, besides other cellular aberrations. Studies on the mechanisms underlying these changes have unraveled the involvement of mitogen-activated protein kinase (MAPK) pathway in the disease process. We describe here a gene expression- and bioinformatics-based study showing that celastrol, a natural triterpenoid, acting via MAPK pathway regulates the downstream genes encoding serum/glucocorticoid regulated kinase 1 (SGK1), which plays a vital role in Th17/Treg differentiation, and brain-derived neurotrophic factor (BDNF), which is a neurotrophic factor, thereby offering protection against experimental autoimmune encephalomyelitis (EAE) in mice.
Methods
We first tested the gene expression profile of splenocytes of EAE mice in response to the disease-related antigen, myelin oligodendrocyte glycoprotein (MOG), and then examined the effect of celastrol on that profile.
Results
Interestingly, celastrol reversed the expression of many MOG-induced genes involved in inflammation and immune pathology. The MAPK pathway involving p38MAPK and ERK was identified as one of the mediators of celastrol action. It involved suppression of SGK1 but upregulation of BDNF, which then contributed to protection against EAE.
Conclusion
Our results not only provide novel insights into disease pathogenesis, but also offer promising therapeutic targets for MS.





Similar content being viewed by others
Abbreviations
- AP-1:
-
Activator protein 1
- BDNF:
-
Brain-derived neurotrophic factor
- CEBPB:
-
CCAAT enhancer binding protein β1
- ERK1/2:
-
Extracellular signal-regulated kinase ½
- GSK3B:
-
Glycogen synthase kinase 3 beta
- IKBKB:
-
Inhibitor of Nuclear factor kappa B kinase subunit beta
- IRF8:
-
Interferon regulatory factor 8
- MAPK:
-
Mitogen-activated protein kinase
- MOG:
-
Myelin oligodendrocyte glycoprotein
- NFKB1:
-
Nuclear factor kappa B subunit 1
- NFKBIA:
-
NFKB inhibitor alpha
- NR3C1:
-
Nuclear receptor subfamily 3, group C, member 1/glucocorticoid receptor
- PKC:
-
Protein kinase C
- RELA:
-
Rel-like domain-containing proteins
- SGK1:
-
Serum/glucocorticoid regulated kinase 1
- SP1:
-
Specificity protein 1
- STAT4:
-
Signal transducer and activator of transcription 4
- TLR:
-
Toll-like receptor
References
Bhise V, Dhib-Jalbut S. Further understanding of the immunopathology of multiple sclerosis: impact on future treatments. Expert Rev Clin Immunol. 2016;12(10):1069–89.
Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17(Suppl 5 Improving):139–45.
Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86(11):1014–21.
Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol. 2015;28(3):206–19.
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–7.
Holmoy T. The immunology of multiple sclerosis: disease mechanisms and therapeutic targets. Minerva Med. 2008;99(2):119–40.
Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4(7):384–98.
Di Mitri D, Sambucci M, Loiarro M, De Bardi M, Volpe E, Cencioni MT, Gasperini C, Centonze D, Sette C, Akbar AN, et al. The p38 mitogen-activated protein kinase cascade modulates T helper type 17 differentiation and functionality in multiple sclerosis. Immunology. 2015;146(2):251–63.
Lochner M, Wang Z, Sparwasser T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog Mol Biol Transl Sci. 2015;136:99–129.
Wang C, Collins M, Kuchroo VK. Effector T cell differentiation: are master regulators of effector T cells still the masters? Curr Opin Immunol. 2015;37:6–10.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Ann Rev Immunol. 2009;27:485–517.
Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–76.
Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Ann Rev Immunol. 2012;30:531–64.
Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 2017;22(1):134.
Fessler J, Felber A, Duftner C, Dejaco C. Therapeutic potential of regulatory T cells in autoimmune disorders. BioDrugs. 2013;27(4):281–91.
Mai J, Wang H, Yang XF. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci (Landmark Ed). 2010;15:986–1006.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–22.
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7.
Krementsov DN, Thornton TM, Teuscher C, Rincon M. The emerging role of p38 mitogenactivated protein kinase in multiple sclerosis and its models. Mol Cell Biol. 2013;33(19):3728–34.
Canto E, Isobe N, Didonna A, Hauser SL, Oksenberg JR. Aberrant STAT phosphorylation signaling in peripheral blood mononuclear cells from multiple sclerosis patients. J Neuroinflammation. 2018;15(1):72.
Huang G, Wang Y, Vogel P, Chi H. Control of IL-17 receptor signaling and tissue inflammation by the p38alpha-MKP-1 signaling axis in a mouse model of multiple sclerosis. Sci Signal. 2015;8(366):ra24.
Krementsov DN, Noubade R, Dragon JA, Otsu K, Rincon M, Teuscher C. Sex-specific control of central nervous system autoimmunity by p38 mitogen-activated protein kinase signaling in myeloid cells. Ann Neurol. 2014;75(1):50–66.
Namiki K, Matsunaga H, Yoshioka K, Tanaka K, Murata K, Ishida J, Sakairi A, Kim J, Tokuhara N, Shibakawa N, et al. Mechanism for p38alpha-mediated experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287(29):24228–38.
Noubade R, Krementsov DN, Del Rio R, Thornton T, Nagaleekar V, Saligrama N, Spitzack A, Spach K, Sabio G, Davis RJ, et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118(12):3290–300.
Wang L, Li B, Quan MY, Li L, Chen Y, Tan GJ, Zhang J, Liu XP, Guo L. Mechanism of oxidative stress p38MAPK-SGK1 signaling axis in experimental autoimmune encephalomyelitis (EAE). Oncotarget. 2017;8(26):42808–16.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et biophysica acta. 2010;1802(4):396–405.
Astry B, Venkatesha SH, Laurence A, Christensen-Quick A, Garzino-Demo A, Frieman MB, O’Shea JJ, Moudgil KD. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol. 2015;157(2):228–38.
Nanjundaiah SM, Venkatesha SH, Yu H, Tong L, Stains JP, Moudgil KD. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J Biol Chem. 2012;287(26):22216–26.
Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathogens Dis 2016;74(6):ftw059.
Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286(17):15138–46.
Abdin AA, Hasby EA. Modulatory effect of celastrol on Th1/Th2 cytokines profile, TLR2 and CD3 + T-lymphocyte expression in a relapsing-remitting model of multiple sclerosis in rats. Eur J Pharmacol. 2014;742:102–12.
Wang Y, Cao L, Xu LM, Cao FF, Peng B, Zhang X, Shen YF, Uzan G, Zhang DH. Celastrol ameliorates EAE induction by suppressing pathogenic T cell responses in the peripheral and central nervous systems. J Neuroimmune Pharmacol. 2015;10(3):506–16.
Yang H, Liu C, Jiang J, Wang Y, Zhang X. Celastrol attenuates multiple sclerosis and optic neuritis in an experimental autoimmune encephalomyelitis model. Front Pharmacol. 2017;8:44.
Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014;86:e51275.
Kwong B, Rua R, Gao Y, Flickinger J Jr, Wang Y, Kruhlak MJ, Zhu J, Vivier E, McGavern DB, Lazarevic V. T-bet-dependent NKp46(+) innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol. 2017;18(10):1117–27.
Wu C, Chen Z, Xiao S, Thalhamer T, Madi A, Han T, Kuchroo V. SGK1 governs the reciprocal development of Th17 and regulatory T cells. Cell Rep. 2018;22(3):653–65.
Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11(6):1164–78.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736.
Venkatesha SH, Moudgil KD. Celastrol and its role in controlling chronic diseases. Adv Exp Med Biol. 2016;928:267–89.
Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303(1):9–20.
Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun. 2010;394(3):439–42.
Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009;10:109.
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y, Meng G, Xie L, Wang J, Xiao Y, et al. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One. 2013;8(6):e65477.
Cascao R, Fonseca JE, Moita LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med (Lausanne). 2017;4:69.
Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–34.
Choi BS, Kim H, Lee HJ, Sapkota K, Park SE, Kim S, Kim SJ. Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson’s disease. Neurochem Res. 2014;39(1):84–96.
Luo D, Guo Y, Cheng Y, Zhao J, Wang Y, Rong J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-kappaB pathways. Aging (Albany NY). 2017;9(10):2069–82.
Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125(11):4212–22.
van der Meer JW, Netea MG. A salty taste to autoimmunity. The New England journal of medicine. 2013;368(26):2520–1.
BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Gorlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res. 2006;98(6):828–36.
Pastore D, Della-Morte D, Coppola A, Capuani B, Lombardo MF, Pacifici F, Ferrelli F, Arriga R, Mammi C, Federici M, et al. SGK-1 protects kidney cells against apoptosis induced by ceramide and TNF-alpha. Cell Death Dis. 2015;6:e1890.
Bell LM, Leong ML, Kim B, Wang E, Park J, Hemmings BA, Firestone GL. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J Biol Chem. 2000;275(33):25262–72.
De Santi L, Annunziata P, Sessa E, Bramanti P. Brain-derived neurotrophic factor and TrkB receptor in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neurol Sci. 2009;287(1–2):17–26.
Stadelmann C, Kerschensteiner M, Misgeld T, Bruck W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125(Pt 1):75–85.
Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29(1):43–68.
Smith PA, Schmid C, Zurbruegg S, Jivkov M, Doelemeyer A, Theil D, Dubost V, Beckmann N. Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J Neuroimmunol. 2018;318:103–13.
Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24(4):641–51.
Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–42.
Veerappan K, Natarajan S, Ethiraj P, Vetrivel U, Samuel S. Inhibition of IKKbeta by celastrol and its analogues - an in silico and in vitro approach. Pharm Biol. 2017;55(1):368–73.
Qiu X, Luo H, Liu X, Guo Q, Zheng K. Fan D1, Shen J. Lu C1, He X, Zhang G, Lu A. Therapeutic Potential of Pien Tze Huang on Experimental AutoimmuneEncephalomyelitis Rat. J Immunol Res. 2018;2018:2952471
Yang L, Xing F, Han X, Li Q, Wu H, Shi H, Wang Z, Huang F, Wu X. Astragaloside IV regulates differentiation and induces apoptosis of activated CD4+ T cells in the pathogenesis of experimental autoimmune encephalomyelitis. Toxicol Appl Pharmacol. 2018;362:105–115.
Acknowledgements
This work was supported in part by Grants (1R21NS082918 and R01 AT 004321) from the National Institutes of Health, Bethesda, MD and in part by VA Merit Review Award # 5 I01 BX002424 (to KDM) from the United States (U.S.) Department of Veterans Affairs [Biomedical Laboratory Research and Development Service]. We thank Jason Lees (USUHS, Bethesda) and Bodhraj Acharya (UMB) for helpful advice and discussion regarding the MOG-EAE model. We thank Rakeshchandra Reddy Meka and Steven Dudics for help with some experiments. We also thank Carol Fowler and Tom Bowen for help with the VA Research Facilities. This material is the result of work supported in part with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Additional information
Responsible Editor: John Di Battista.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Venkatesha, S.H., Moudgil, K.D. Celastrol suppresses experimental autoimmune encephalomyelitis via MAPK/SGK1-regulated mediators of autoimmune pathology. Inflamm. Res. 68, 285–296 (2019). https://doi.org/10.1007/s00011-019-01219-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01219-x