Abstract
Objective and design
The receptor for advanced glycation endproducts (RAGE) is an innate immunity receptor that has been implicated in the pathogenesis of atherosclerotic cardiovascular disease. However, the possibility that RAGE-mediated signaling is involved in angiotensin II (Ang II)-induced cardiac left ventricular hypertrophy has yet to be investigated. We therefore determined whether RAGE has a role in regulating pathological cardiac hypertrophy.
Materials and subjects
Protein abundance was estimated using Western blotting and intracellular ROS level and phospho-p65 were detected using fluorescence microscopy. Enzyme-linked immunosorbent assay was used to detect HMGB1 and IL-1β. All in vitro experiments were performed using H9C2 cells.
Treatments
To induce cardiomyocyte hypertrophy, 300 nM Ang II was treated for 48 h and 2 µg/ml sRAGE was treated 1 h prior to addition of Ang II.
Results
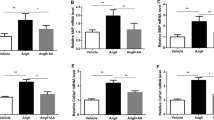
sRAGE attenuated Ang II-induced cardiomyocyte hypertrophy by downregulating RAGE and angiotensin II type 1 receptor expression. Secretion levels of high motility group box 1 and interleukin-1β, estimated from a cell culture medium, were significantly reduced by sRAGE. Activated PKCs and ERK1/2, important signals in left ventricular hypertrophy (LVH) development, were downregulated by sRAGE treatment. Furthermore, we found that nuclear factor-κB and NOD-like receptor protein 3 (NLRP3) were associated with RAGE-mediated cardiomyocyte hypertrophy.
Conclusions
In the context of these results, we conclude that RAGE induces cardiac hypertrophy through the activation of the PKCs-ERK1/2 and NF-κB-NLRP3-IL1β signaling pathway, and suggest that RAGE-NLRP3 may be an important mediator of Ang II-induced cardiomyocyte hypertrophy. In addition, we determined that inhibition of RAGE activation with soluble RAGE (sRAGE) has a protective effect on Ang II-induced cardiomyocyte hypertrophy.





Similar content being viewed by others
Abbreviations
- AT1R:
-
Angiotensin II receptor type I
- RAGE:
-
Receptor for advanced glycation endproducts
- Ang II:
-
Angiotensin II
- NLRP3:
-
Nucleotide-binding oligomerization domain-like receptor protein 3
- sRAGE:
-
Soluble RAGE
- LVH:
-
Left ventricular hypertrophy
- ROS:
-
Reactive oxygen species
- HMGB1:
-
High-mobility group box 1
References
Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA. 2000;97(2):931–6. https://doi.org/10.1073/pnas.97.2.931.
Matsui T, Yamagishi S, Ueda S, Nakamura K, Imaizumi T, Takeuchi M, et al. Telmisartan, an angiotensin II type 1 receptor blocker, inhibits advanced glycation end-product (AGE)-induced monocyte chemoattractant protein-1 expression in mesangial cells through downregulation of receptor for AGEs via peroxisome proliferator-activated receptor-gamma activation. J Int Med Res. 2007;35(4):482–9. https://doi.org/10.1177/147323000703500407.
Lin L, Park S, Lakatta EG. RAGE signaling in inflammation and arterial aging. Front Biosci. 2009;14:1403–13.
Park S, Yoon SJ, Tae HJ, Shim CY. RAGE and cardiovascular disease. Front Biosci. 2011;16:486–97.
Kang R, Tang DL, Lotze MT, Zeh HJ. RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy. 2011;7(4):442–4. https://doi.org/10.4161/auto.7.4.14681.
Hariharan N, Ikeda Y, Hong C, Alcendor RR, Usui S, Gao SM, et al. Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. Plos One. 2013. https://doi.org/10.1371/journal.pone.0051632.
Yan L, Mathew L, Chellan B, Gardner B, Earley J, Puri TS, et al. S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler Thromb Vasc Biol. 2014;34(7):1399–411. https://doi.org/10.1161/ATVBAHA.114.303508.
Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–7. https://doi.org/10.1038/ni.1703.
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–25.
Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2 × 7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175(11):7611–22.
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275(5297):206–9.
Orn S, Ueland T, Manhenke C, Sandanger O, Godang K, Yndestad A, et al. Increased interleukin-1beta levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med. 2012;272(3):267–76. https://doi.org/10.1111/j.1365-2796.2012.02517.x.
Harada E, Nakagawa O, Yoshimura M, Harada M, Nakagawa M, Mizuno Y, et al. Effect of interleukin-1 beta on cardiac hypertrophy and production of natriuretic peptides in rat cardiocyte culture. J Mol Cell Cardiol. 1999;31(11):1997–2006. https://doi.org/10.1006/jmcc.1999.1030.
Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol. 2013;98(2):462–72. https://doi.org/10.1113/expphysiol.2012.068338.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357-U7. https://doi.org/10.1038/Nature08938.
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011;108(49):19725–30. https://doi.org/10.1073/pnas.1108586108.
Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, et al. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156(11):4281–92. https://doi.org/10.1210/en.2015-1408.
Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL, et al. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin. 2015;36(7):821–30. https://doi.org/10.1038/aps.2015.21.
Watson AM, Li J, Samijono D, Bierhaus A, Thomas MC, Jandeleit-Dahm KA, et al. Quinapril treatment abolishes diabetes-associated atherosclerosis in RAGE/apolipoprotein E double knockout mice. Atherosclerosis. 2014;235(2):444–8. https://doi.org/10.1016/j.atherosclerosis.2014.05.945.
Tae HJ, Kim JM, Park S, Tomiya N, Li G, Wei W, et al. The N-glycoform of sRAGE is the key determinant for its therapeutic efficacy to attenuate injury-elicited arterial inflammation and neointimal growth. J Mol Med. 2013;91(12):1369–81. https://doi.org/10.1007/s00109-013-1091-4.
Yang WI, Lee D, Lee DL, Hong SY, Lee SH, Kang SM, et al. Blocking the receptor for advanced glycation end product activation attenuates autoimmune myocarditis. Circ J. 2014;78(5):1197-U216. https://doi.org/10.1253/circj.CJ-13-1235.
Liu Q, Chen HB, Luo M, Zheng H. Serum soluble RAGE level inversely correlates with left ventricular hypertrophy in essential hypertension patients. Genet Mol Res. 2016. https://doi.org/10.4238/gmr.15028414.
Chen J, Zhang J, Xu L, Xu C, Chen S, Yang J, et al. Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis. 2012;224(2):332–9. https://doi.org/10.1016/j.atherosclerosis.2012.07.020.
Polizio AH, Balestrasse KB, Yannarelli GG, Noriegai GO, Gorzalczany S, Taira C, et al. Angiotensin II regulates cardiac hypertrophy via oxidative stress but not antioxidant enzyme activities in experimental renovascular hypertension. Hypertens Res. 2008;31(2):325–34. https://doi.org/10.1291/hypres.31.325.
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20(4):742–52. https://doi.org/10.1681/Asn.2008050514.
Gawdzik J, Mathew L, Kim G, Puri TS, Bowman MAH. Vascular remodeling and arterial calcification are directly mediated by S100A12 (EN-RAGE) in chronic kidney disease. Am J Nephrol. 2011;33(3):250–9. https://doi.org/10.1159/000324693.
Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity—upstream mediators. Circ Res. 2002;91(5):406–13. https://doi.org/10.1161/01.Res.0000033523.08033.16.
Ihara Y, Egashira K, Nakano K, Ohtani K, Kubo M, Koga J, et al. Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol. 2007;43(4):455–64. https://doi.org/10.1016/j.yjmcc.2007.07.044.
Lee TW, Kao YH, Lee TI, Chang CJ, Lien GS, Chen YJ. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol. 2014;173(2):236–41. https://doi.org/10.1016/j.ijcard.2014.02.041.
Lin H, Shen L, Zhang X, Xie J, Hao H, Zhang Y, et al. HMGB1-RAGE axis makes no contribution to cardiac remodeling induced by pressure-overload. PLoS One. 2016;11(6):e0158514. https://doi.org/10.1371/journal.pone.0158514.
Nair AR, Ebenezer PJ, Saini Y, Francis J. Angiotensin II-induced hypertensive renal inflammation is mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial cells. Exp Cell Res. 2015;335(2):238–47. https://doi.org/10.1016/j.yexcr.2015.05.011.
Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology. 2010;215(12):956–62. https://doi.org/10.1016/j.imbio.2009.11.001.
Funayama A, Shishido T, Netsu S, Narumi T, Kadowaki S, Takahashi H, et al. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2013;99(4):657–64. https://doi.org/10.1093/cvr/cvt128.
Petersen CA, Burleigh BA. Role for interleukin-1 beta in Trypanosoma cruzi-induced cardiomyocyte hypertrophy. Infect Immun. 2003;71(8):4441–7.
Koulis C, Watson AM, Gray SP, Jandeleit-Dahm KA. Linking RAGE and Nox in diabetic micro- and macrovascular complications. Diabetes Metab. 2015;41(4):272–81. https://doi.org/10.1016/j.diabet.2015.01.006.
Min HJ, Kim JH, Yoo JE, Oh JH, Kim KS, Yoon JH, et al. ROS-dependent HMGB1 secretion upregulates IL-8 in upper airway epithelial cells under hypoxic condition. Mucosal Immunol. 2017;10(3):685–94. https://doi.org/10.1038/mi.2016.82.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. https://doi.org/10.1038/Nature00858.
Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. https://doi.org/10.1111/j.1600-065X.2007.00574.x.
Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183(3):2008–15. https://doi.org/10.4049/jimmunol.0900138.
Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J Neuroinflamm. 2015;12:137. https://doi.org/10.1186/s12974-015-0360-2.
De Batista PR, Palacios R, Martin A, Hernanz R, Medici CT, Silva MA, et al. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One. 2014;9(8):e104020. https://doi.org/10.1371/journal.pone.0104020.
Wang L, Li YL, Zhang CC, Cui W, Wang X, Xia Y, et al. Inhibition of Toll-like receptor 2 reduces cardiac fibrosis by attenuating macrophage-mediated inflammation. Cardiovasc Res. 2014;101(3):383–92. https://doi.org/10.1093/cvr/cvt258.
Gasiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J. RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol Neurobiol. 2017. https://doi.org/10.1007/s12035-017-0419-4.
Trentin-Sonoda M, da Silva RC, Kmit FV, Abrahao MV, Monnerat Cahli G, Brasil GV, et al. Knockout of toll-like receptors 2 and 4 prevents renal ischemia-reperfusion-induced cardiac hypertrophy in mice. PLoS One. 2015;10(10):e0139350. https://doi.org/10.1371/journal.pone.0139350.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1A2A2A01007346) and (NRF-2015R1C1A1A01054945), and by the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (HI08C2149).
Author information
Authors and Affiliations
Contributions
MEL mainly participated in all experiments and editing manuscript and SL and SP mainly conceive the study and wrote the manuscript; JJ, JL, and SC performed the experiment and analyses; MS participated in the study design. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Availability of data and material
All data supporting the conclusions of this article are included within the article.
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Andrew Roberts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, S., Lee, M.E., Jeong, J. et al. sRAGE attenuates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting RAGE-NFκB-NLRP3 activation. Inflamm. Res. 67, 691–701 (2018). https://doi.org/10.1007/s00011-018-1160-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1160-9