Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic and progressive lung disease characterized by a mixture of small airway disease and lung tissue parenchymal destruction. Abnormal inflammatory responses to cigarette smoking and other noxious particles are generally thought to be responsible for causing of COPD. Since airway inflammation is a key factor in COPD progress, it is crucial to unravel its underlying molecular mechanisms. Unbiased analysis of genome-wide gene expression profiles in lung small airway epithelial cells provides a powerful tool to investigate this.
Methods
Gene expression data of GSE611906, GSE20257, GSE8545 were downloaded from GEO database. All 288 lung small airway samples in these cohorts, including donors with (n = 61) and without (n = 227) COPD, were chosen for differential gene expression analysis. The gene ontology (GO) function, Kyoto Encyclopedia of Genes and Genomes pathway (KEGG) enrichment analyses, gene co-expression network analysis (WGCNA) and protein–protein interaction (PPI) network analysis were performed. Subsequently, the analyses of IL1B expression level, the Pearson correlation between IL1B and several COPD biomarkers were performed using other cohorts to validate our main findings.
Results
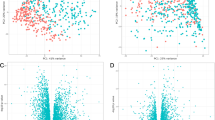
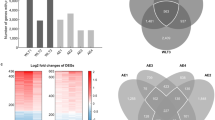
With a change ≥ twofold and P value < 0.05 cutoff, we found 38 genes were up-regulated and 114 genes were down-regulated in patients with COPD compared with health controls, while using cutoff fold change 1.5 and P value < 0.05, there were 318 genes up-regulated and 333 genes down-regulated. Among the most up-regulated genes were IL1B, CCL2, CCL23, and CXCL14, all implicated in inflammation triggering. GO, KEGG and WGCNA analysis all disclosed IL1B was highly correlated to COPD disease trait. The expression profile of IL1B was further validated using independent cohorts from COPD airway epithelium, lung tissue, sputum, and blood. We demonstrated higher IL1B gene expression in COPD small airway epithelial cells, but not in COPD lung tissue, sputum, and blood. Strong co-expression of IL1B with COPD biomarkers, such as DUOX2, MMP12, CCL2, and CXCL14, were validated in silico analysis. Finally, PPI network analysis using enriched data showed IL1B, CCL2, CCL7 and BMP7 were in the same hub node with high degrees.
Conclusions
We identified IL1B was significantly up-regulated in COPD small airway epithelial cells and propose IL1B as a novel player in airway inflammation in COPD.








Similar content being viewed by others
Abbreviations
- IL1B:
-
Interleukin 1 beta
- IL1R2:
-
Interleukin-1 receptor type 2
- COPD:
-
Chronic obstructive pulmonary disease
- CCL2:
-
Chemokine (C-C motif) ligand 2
- CCL7:
-
Chemokine (C-C motif) ligand 7
- CCL23:
-
Chemokine (C-C motif) ligand 23
- HIF-1α:
-
Hypoxia inducible factor-1 alpha
- DEGs:
-
Differentially expressed genes
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- ALDH3A1:
-
Aldehyde dehydrogenase 3 family, member A1
- DUOX2:
-
Dual oxidase 2
- MMP12:
-
Matrix metalloproteinase 12
- AKR1B10:
-
Aldo–keto reductase family 1, member B10
- CYP1B1:
-
Cytochrome P450 family 1 subfamily B polypeptide 1
- NQO1:
-
NAD(P)H:quinoneoxidoreductase
- PPI:
-
Protein–protein interaction
- GS:
-
Gene significance
- MM:
-
Module membership
- GSEA:
-
Gene set enrichment analysis
- IL17:
-
Interleukin-17
- P450:
-
Cytochrome P450
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- WGCNA:
-
Weighted Gene Co-expression Network Analysis
References
Diaz-Guzman E, Mannino DM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:7–16.
Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Ann Rev Pathol 2009;4:435–59.
McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–75.
Tonnesen P. Smoking cessation. and COPD. Eur Respir Rev Off J Eur Respir Soc. 2013;22:37–43.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3). https://doi.org/10.1183/13993003.00214-2017.
Goncalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60:409–24.
Kim WD, Ling SH, Coxson HO, English JC, Yee J, Levy RD, et al. The association between small airway obstruction and emphysema phenotypes in COPD. Chest. 2007;131:1372–8.
Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143:1436–43.
Hogg JC, Pare PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97:529–52.
Angelis N, Porpodis K, Zarogoulidis P, Spyratos D, Kioumis I, Papaiwannou A, et al. Airway inflammation in chronic obstructive pulmonary disease. J Thorac Dis. 2014;6(Suppl 1):S167–72.
Yang J, Zuo WL, Fukui T, Chao I, Gomi K, Lee B, et al. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am J Respir Crit Care Med. 2017;196:340–52.
Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, et al. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci CMLS 2011;68:877–92.
Raman T, O’Connor TP, Hackett NR, Wang W, Harvey BG, Attiyeh MA, et al. Quality control in microarray assessment of gene expression in human airway epithelium. BMC Genom. 2009;10:493.
Ammous Z, Hackett NR, Butler MW, Raman T, Dolgalev I, O’Connor TP, et al. Variability in small airway epithelial gene expression among normal smokers. Chest. 2008;133:1344–53.
Morrow JD, Zhou X, Lao T, Jiang Z, DeMeo DL, Cho MH, et al. Functional interactors of three genome-wide association study genes are differentially expressed in severe chronic obstructive pulmonary disease lung tissue. Sci Rep. 2017;7:44232.
Singh D, Fox SM, Tal-Singer R, Plumb J, Bates S, Broad P, et al. Induced sputum genes associated with spirometric and radiological disease severity in COPD ex-smokers. Thorax. 2011;66:489 – 95.
Davies C, Rhodes JA, Barnes P, Donnelly L. Elevated CCL2 responses in COPD and attenuation by selective chemokine receptor antagonists. Eur Respir J. 2015;46(suppl 59):PA3900.
Abdel-Halim M, Darwish SS, ElHady AK, Hoppstadter J, Abadi AH, Hartmann RW, et al. Pharmacological inhibition of protein kinase C (PKC)zeta downregulates the expression of cytokines involved in the pathogenesis of chronic obstructive pulmonary disease (COPD). Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2016;93:405–9.
Qin S, Huleihel L, Lucht L, Clarke A, Ries JW, Kessinger C, et al. Alterations of inflammatory chemokine and matrix metalloproteinase mRNA levels in BAL cells from HIV-infected COPD patients. Am J Resp Crit Care. 2015;191:A4716.
Frankenberger M, Eder C, Hofer TP, Heimbeck I, Skokann K, Kassner G, et al. Chemokine expression by small sputum macrophages in COPD. Mol Med. 2011;17:762–70.
Eapen MS, Myers S, Walters EH, Sohal SS. Airway inflammation in chronic obstructive pulmonary disease (COPD): a true paradox. Expert Rev Respir Med. 2017;11:827–39.
Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res Off J Eur Histamine Res Soc [et al]. 2008;57:497–503.
Vogelmeier C, Koczulla R, Fehrenbach H, Bals R. Pathogenesis of chronic obstructive pulmonary disease. Der Internist. 2006;47(6):885–6 (888–90, 892–4).
Szulakowski P, Mroz RM, Pierzchala W, Chyczewska E, MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Molecular mechanisms (part II). Wiad Lek. 2006;59:250–4.
McGuinness AJ, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. 2017;6(2):21.
Choudhury G, MacNee W. Role of inflammation and oxidative stress in the pathology of ageing in COPD: potential therapeutic interventions. Copd. 2017;14:122–35.
Malic Z, Topic A, Francuski D, Stankovic M, Nagorni-Obradovic L, Markovic B, et al. Oxidative stress and genetic variants of xenobiotic-metabolising enzymes associated with copd development and severity in serbian adults. Copd. 2017;14:95–104.
Meihua G, Jian W, Nanshan Z. [esearch progress of the role of oxidative stress in the pathogenesis of COPD. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chin J Tuberc Respir Dis. 2015;38:222–4.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12:543–59.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. gold executive summary. Am J Respir Crit Care Med. 2017;195:557–82.
Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respir Int Rev Thorac Dis. 2010;80:89–95.
Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–41.
Xie ZK, Huang QP, Huang J, Xie ZF. Association between the IL1B, IL1RN polymorphisms and COPD risk: a meta-analysis. Sci Rep. 2014;4:6202.
Mei JJ, Liang Y, Shen N, He B. Association between interleukin-1B polymorphisms and chronic obstructive pulmonary disease: a meta-analysis. Zhonghua yi xue za zhi. 2013;93:910–5.
Danilko KV, Korytyna GF, Akhmadishina LZ, Yanbaeva DG, Zagidullin SZ, Victorova TV. Association of polymorphisms of cytokine genes (IL1B, IL1RN, TNFA, LTA, IL6, IL8, and IL10) with chronic obstructive pulmonary disease. Mol Biol. 2007;41:22–31.
Mahajan B, Vijayan VK, Agarwal MK, Bansal SK. Serum interleukin-1beta as a marker for differentiation of asthma and chronic obstructive pulmonary disease. Biomarkers. 2008;13:713–27.
Hammad DR, Elgazzar AG, Essawy TS, Sameie SA. Evaluation of serum interleukin-1 beta as an inflammatory marker in copd patients. Egypt J Chest Dis Tuberc. 2015;64:347–52.
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Ann Rev Immunol. 2009;27:519–50.
Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–52.
Hellermann GR, Nagy SB, Kong X, Lockey RF, Mohapatra SS. Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir Res. 2002;3:22.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, et al. Succinate is an inflammatory signal that induces il-1β through hif-1α. Nature. 2013;496(7444):238–42.
Hashimoto K, Oreffo RO, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60:3303–13.
Acknowledgements
We thank all members from department of central laboratory, the Fifth Affiliated Hospital of Guangzhou Medical University for their invaluable help.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number. 81400013), Science and Technology Planning Project of Guangdong Province, China (Grant Number. 2014A20212329) and Department of education of GuangDong Province, China (Grant Number. 2016KTSCX110).
Author information
Authors and Affiliations
Contributions
ZYL, XKZ and JFL designed the study; ML (Min Liang), ML (Ming Li) and XMF performed data collection; GY and YXL analyzed the data; ZYL and GY wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
In the current study, all analyses were based on publicly available data, and this article does not contain any studies with human participants and animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Liwu Li.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, G., Liang, M., Li, M. et al. A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflamm. Res. 67, 539–551 (2018). https://doi.org/10.1007/s00011-018-1145-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1145-8