Abstract
Objective and design
The goal of this study was to assess the capacity of VBP15, a dissociative steroidal compound, to reduce pro-inflammatory cytokine expression in vitro, to reduce symptoms of colitis in the trinitrobenzene sulfonic acid-induced murine model, and to assess the effect of VBP15 on growth stunting in juvenile mice.
Materials
In vitro studies were performed in primary human intestinal epithelial cells. Colitis was induced in mice by administering trinitrobenzene sulfonic acid. Growth stunting studies were performed in wild type outbred mice.
Treatment
Cells were treated with VBP15 or prednisolone (10 μM) for 24 h. Mice were subjected to 3 days of VBP15 (30 mg/kg) or prednisolone (30 mg/kg) in the colitis study. In the growth stunting study, mice were subjected to VBP15 (10, 30, 45 mg/kg) or prednisolone (10 mg/kg) for 5 weeks.
Methods
Cytokines were measured by PCR and via Luminex. Colitis symptoms were evaluated by assessing weight loss, intestinal blood, and stool consistency. Growth stunting was assessed using an electronic caliper.
Results
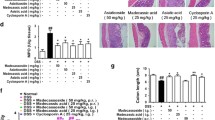
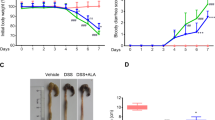
VBP15 significantly reduced the in vitro production of CCL5 (p < 0.001) IL-6 (p < 0.001), IL-8 (p < 0.05) and reduced colitis symptoms (p < 0.05). VBP15 caused less growth stunting than prednisolone (p < 0.001) in juvenile mice.
Conclusion
VBP15 may reduce symptoms of IBD, while decreasing or avoiding detrimental side effects.






Similar content being viewed by others
References
Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. 2013;5:237–47.
Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn’s disease. Aliment Pharmacol Ther. 2001;15:1515–25.
Moeeni V, Day AS. Impact of inflammatory bowel disease upon growth in children and adolescents. ISRN Pediatr. 2011;2011:365712.
Mrakotsky C, Forbes PW, Bernstein JH, Grand RJ, Bousvaros A, Szigethy E, Waber DP. Acute cognitive and behavioral effects of systemic corticosteroids in children treated for inflammatory bowel disease. J Int Neuropsychol Soc. 2013;19:96–109.
Faubion W, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60.
Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–28.
Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380:41–54.
Baudy AR, Saxena N, Gordish H, Hoffman EP, Nagaraju K. A robust in vitro screening assay to identify NF-kappaB inhibitors for inflammatory muscle diseases. Int Immunopharmacol. 2009;9:1209–14.
Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459–79.
van der Burg B, Liden J, Okret S, Delaunay F, Wissink S, van der Saag PT, Gustafsson JA. Nuclear factor-kappa B repression in antiinflammation and immunosuppression by glucocorticoids. Trends Endocrinol Metab. 1997;8:152–7.
Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–41.
Reichardt HM, Tuckermann JP, Göttlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schütz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20:7168–73.
Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–6.
Neurath MF, Pettersson S. Meyer zum Büschenfelde KH. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004.
Tang Y, Clayburgh DR, Mittal N, Goretsky T, Dirisina R, Zhang Z, Kron M, Ivancic D, Katzman RB, Grimm G, Lee G, Fryer J, Nusrat A, Turner JR, Barrett TA. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol. 2010;176:158–67.
Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90.
Thiele K, Bierhaus A, Autschbach F, Hofmann M, Stremmel W, Thiele H, Ziegler R, Nawroth PP. Cell specific effects of glucocorticoid treatment on the NF κBp65/IκBα system in patients with Crohn’s disease. Gut. 1999;45:693–704.
De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W. Haegeman. Glucocorticoid-mediated repression of nuclear factor-κB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–9.
Reeves EK, Hoffman EP, Nagaraju K, Damsker JM, McCall JM. VBP15: preclinical characterization of a novel anti-inflammatory delta 9,11 steroid. Bioorg Med Chem. 2013;21:2241–9.
Damsker JM, Dillingham BC, Rose MC, Balsley MA, Heier CR, Watson AM, Stemmy EJ, Jurjus RA, Huynh T, Tatem K, Uaesoontrachoon K, Berry DM, Benton AS, Freishtat RJ, Hoffman EP, McCall JM, Gordish-Dressman H, Constant SL, Reeves EK, Nagaraju K. VBP15, a glucocorticoid analogue, is effective at reducing allergic lung inflammation in mice. PLoS One. 2013;8:e63871.
Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5:1569–85.
Dillingham BC, Knoblach SM, Many GM, Harmon BT, Mullen AM, Heier CR, Bello L, McCall JM, Hoffman EP, Connor EM, Nagaraju K, Reeves EK, Damsker JM. VBP15, a novel anti-inflammatory, is effective at reducing the severity of murine experimental autoimmune encephalomyelitis. Cell Mol Neurobiol. 2015;35:377–87.
Reuter KC, Grunwitz CR, Kaminski BM, Steinhilber D, Radeke HH, Stein J. Selective glucocorticoid receptor agonists for the treatment of inflammatory bowel disease: studies in mice with acute trinitrobenzene sulfonic acid colitis. J Pharmacol Exp Ther. 2012;341:68–80.
Scheiffele F, Fuss IV. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002;Chapter 15:Unit 15.19. doi:10.1002/0471142735.im1519s49.
Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9(12):e114195.
Kucharzik T, Hudson JT 3rd, Lügering A, Abbas JA, Bettini M, Lake JG, Evans ME, Ziegler TR, Merlin D, Madara JL, Williams IR. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54(11):1565–72.
Yang SK, Choi MS, Kim OH, Myung SJ, Jung HY, Hong WS, Kim JH, Min YI. The increased expression of an array of C-X-C and C-C chemokines in the colonic mucosa of patients with ulcerative colitis: regulation by corticosteroids. Am J Gastroenterol. 2002;97(1):126–32.
Lillard JW Jr, Boyaka PN, Taub DD, McGhee JR. RANTES potentiates antigen-specific mucosal immune responses. J Immunol. 2001;166(1):162–9.
McCormack G, Moriarty D, O’Donoghue DP, McCormick PA, Sheahan K, Baird AW. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res. 2001;50:491–5.
Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–46.
Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34.
DeBosscher KG, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10:497–504.
Adcock IM, Barnes PJ. Ligand-induced differentiation of glucocorticoid receptor (GR) transrepression and transactivation. Biochem Soc Trans. 1996;24:267S.
Bijsmans IT, Guercini C, Ramos Pittol JM, Omta W, Milona A, Lelieveld D, Egan DA, Pellicciari R, Gioiello A, van Mil SW. The glucocorticoid mometasone furoate is a novel FXR ligand that decreases inflammatory but not metabolic gene expression. Sci Rep. 2015;5:14086.
Robertson S, Allie-Reid F, Vanden Berghe W, Visser K, Binder A, Africander D, Vismer M, De Bosscher K, Hapgood J, Haegeman G, Louw A. Abrogration of glucocorticoid receptor dimerization correlates with dissociated glucocorticoid behavior of compound a. J Biol Chem. 2010;12:8061–8075.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Jesse Damsker and Dr. John McCall are employed by ReveraGen BioPharma Inc. and have stock options and founder shares, respectively.
Financial support
Supported by ReveraGen BioPharma Inc. Sheikh Zayed Institute for Pediatric Surgical Innovation, NIH Grant (1R41DK102235-01).
Additional information
Responsible Editor: John Di Battista.
J. M. Damsker and L. S. Conklin contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Damsker, J.M., Conklin, L.S., Sadri, S. et al. VBP15, a novel dissociative steroid compound, reduces NFκB-induced expression of inflammatory cytokines in vitro and symptoms of murine trinitrobenzene sulfonic acid-induced colitis. Inflamm. Res. 65, 737–743 (2016). https://doi.org/10.1007/s00011-016-0956-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0956-8