Abstract
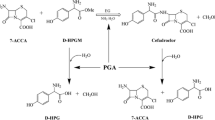
The production of semi-synthetic beta-lactam antibiotics such as Amoxicillin may be performed enzymatically using penicillin acylase under mild conditions. However, the thermodynamically favored hydrolysis of the antibiotic product and the acyl donor substrate needs to be minimized to use the kinetically controlled route. The addition of cosolvents such as ethylene glycol and methanol (the two best solvents identified so far for semi-synthetic beta-lactam antibiotics) can achieve this to some degree, but these additives also produce enzyme inhibition and deactivation. In this study, we compared ethylene glycol and methanol under various substrate conditions. Methanol gave a better synthesis to hydrolysis ratio, although its deactivating effects adversely affected production at lower cosolvent concentrations than ethylene glycol. This effect and its dependence on substrate concentration was further modeled and optimized. A few targets of optimization such as Amoxicllin level, the synthesis to hydrolysis ratio, or a combination, were employed. While maximum levels of Amoxicillin synthesis were achievable only at high substrate concentrations, improvements derived from cosolvents were most significant at low substrate concentrations.
Similar content being viewed by others
References
Ospina, S., E. Barzana, O. T. Ramirez, and A. Lopez-Munguia (1996) Effect of pH in the synthesis of ampicillin by penicillin acylase.Enzyme Microb. Technol. 19: 462–469.
Arroyo, M., I. de la Mata, C. Acebal, and M. P. Castillon (2003) Biotechnological applications of penicillin acylases: state-of-the-art.Appl. Microbiol. Biotechnol. 60: 507–514.
Illanes, A., Z. Cabrera, L. Wilson, and C. Aguirre (2003) Synthesis of cephalexin in ethylene glycol with glyoxylagarose immobilized penicillin acylase: temperature and pH optimization.Process Biochem. 39: 111–117.
Halling, P. J., U. Eichhorn, P. Kuhl, and H.-D. Jakubke (1995) Thermodynamics of solid-to-solid conversion and application to enzymic peptide synthesis.Enzyme Microb. Technol. 17: 601–606.
Ulijn, R. V., A. E. M. Janssen, B. D. Moore, and P. J. Halling (2001) Predicting when precipitation-driven synthesis is feasible: application to biocatalysis.Chem. Eur. J. 7: 2089–2098.
Diender, M. B., A. J. J. Straathof, L. A. M. van der Wielen, C. Ras, and J. J. Heijnen (1998) Feasibility of the thermodynamically controlled synthesis of amoxicillin.J. Mol. Catal. B Enzym. 5: 249–253.
Kim, M. G. and S. B. Lee (1996) Penicillin acylasecatalyzed synthesis of pivampicillin: Effect of reaction variables and organic cosolvents.J. Mol. Catal. B Enzym. 1: 71–80.
Hernandez-Justiz, O., R. Fernandez-Lafuente, M. Terreni, and J. M. Guisan (1998) Use of aquoeus two-phase systems forin situ extraction of water soluble antibiotics during their synthesis by enzymes immobilized on porous supports.Biotechnol. Bioeng. 59: 73–79.
Illanes, A. and A. Fajardo (2001) Kinetically controlled synthesis of ampicillin with immobilized penicillin acylase in the presence of organic solvents.J. Mol. Catal. B Enzym. 11: 587–595.
Yang, L. and D.-Z. Wei (2003) Enhanced enzymatic synthesis of a semi-synthetic cephalosporin, cefaclor. within situ product removal.Biotechnol. Lett. 25: 1195–1198.
Diender, M. B., A. J. J. Straathof, T. van der Does, M. Zomerdijk, and J. J. Heijnen (2000) Course of pH during the formation of amoxicillin by a suspension-to-suspension reaction.Enzyme Microb. Technol. 27: 576–582.
Youshko, M. I., L. M. van Langen, E. de Vroom, F. van Rantwijk, R. A. Sheldon, and V. K. Svedas (2001) Highly efficient synthesis of ampicillin in an “aqueous solution-precipitate” system: repetitive addition of substrates in a semicontinuous process.Biotechnol. Bioeng. 73: 426–430.
Youshko, M. I., H. M. Moody, A. L. Bukhanov, W. H. J. Boosten, and V. K. Svedas (2004) Penicillin acylasecatalyzed synthesis of beta-lactam antibiotics in highly condensed aqueous systems: beneficial impact of kinetic substrate supersaturation.Biotechnol. Bioeng. 85: 323–329.
Basso, A., P. Spizzo, M. Toniutti, c. Ebert, P. Linda, and L. Gardossi (2006) Kinetically controlled synthesis of ampicillin and cephalexin in highly condensed systems in the absence of a liquid aqueous phase.J. Mol. Catal. B Enzym. 39: 105–111.
Fernandez-Lafuente, R., C. M. Rosell, and J. M. Guisan (1991) Enzyme reaction engineering: synthesis of antibiotic catalysed by stabilized penicillin G acylase in the presence of organic cosolvents.Enzyme Microb. Technol. 13: 898–905.
Aguirre, C., J. Baeza, and A. Illanes (1998) Cosolvent effect on the synthesis of ampicillin and cephalexin with penicillin acylase.Prog. Biotechnol. 15: 95–100.
Park, C. B., S. B. Lee, and D. D. Y. Ryu (2000) Penicillin acylase-catalyzed synthesis of cefazolin in water-solvent mixtures: enhancement effect of ethyl acetate and carbon tetrachloride on the synthetic yield.J. Mol. Catal. B Enzym. 9: 275–281.
Arroyo, M., R. Torres-Guzman, I. de la Mata, M. P. Castillon, and C. Acebal (2002) A kinetic examination of penicillin acylase stability in water-organic solvent systems at different temperatures.Biocatal. Biotransform. 20: 53–56.
Wei, D.-Z. and L. Yang (2003) Effects of ethylene glycol on the synthesis of ampicillin using immobilized penicillin G acylase.J. Chem. Technol. Biotechnol. 78: 431–436.
Aguirre, C., M. Toledo, V. Medina, and A. Illanes (2002) Effect of cosolvent and pH on the kinetically controlled synthesis of cephalexin with immobilized penicillin acylase.Process Biochem. 38: 351–360.
Kim, M. G. and S. B. Lee (1996) Effect of organic solvents on penicillin acylase-catalyzed reactions: interaction of organic solvents with enzymes.J. Mol. Catal. B Enzym. 1: 181–190.
Fernandez-Lafuente, R., C. M. Rosell, and J. M. Guisan (1998) The presence of methanol exerts a strong and complex modulation of the synthesis of different antibiotics by immobilized Penicillin G acylase.Enzyme Microb. Technol. 23: 305–310.
Rosell, C. M., M. Terreni, R. Fernandez-Lafuente, and J. M. Guisan (1998) A criterion for the selection of monophasic solvents for enzymatic synthesis.Enzyme Microb. Technol. 23: 64–69.
Arroyo, M., R. Torres-Guzman, I. de la Mata, M. P. Castillon, and C. Acebal (2000) Prediction of penicillin V acylase stability in water-organic co-solvent monophasic systems as a function of solvent composition.Enzyme Microb. Technol. 27: 122–126.
Illanes, A., M. S. Anjari, C. Altamirano, and C. Aguirre (2004) Optimization of cephalexin synthesis with immobilized penicillin acylase in ethylene glycol medium at low temperatures.J. Mol. Catal. B Enzym. 30: 95–103.
Illanes, A., F. Rodriguez, C. Bahamondes, and C. Altamirano (2005) Determination of lumped kinetic parameters and their thermal dependence for the synthesis of cephalexin with immobilized penicillin acylase in organic medium.Biochem. Eng. J. 24: 209–215.
Kim, M. G. and S. B. Lee (1996) Penicillin acylase-catalyzed synthesis of beta-lactam antibiotics in water-methanol mixtures: Effect of cosolvent content and chemical nature of substrate on reaction rates and yields.J. Mol. Catal. B Enzym. 1: 201–211.
Arroyo, M., R. Torres-Guzman, J. Torres-Bacete, I. de la Mata, M. P. Castillon, and C. Acebal (2001) Kinetic mechanism of penicillin V acylase activation by shortchain alcohols.Enzyme Microb. Technol. 29: 312–318.
Chow, Y., J. Wu, and R. Li (2005) Influence of 6-aminopenicillanic acid on amoxicillin synthesis and p-hydroxyphenylglycine methyl ester hydrolysis.Biocatal. Biotransform. 23: 347–351.
Ribeiro, M. P. A., A. L. O. Ferreira, R. L. C. Giordano, and R. C. Giordano (2005) Selectivity of the enzymatic synthesis of ampicillin byE. coli PGA in the presence of high concentrations of substrates.J. Mol. Catal. B Enzym. 33: 81–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chow, Y., Li, R., Wu, J. et al. Modeling and optimization of methanol as a cosolvent in Amoxicillin synthesis and its advantage over ethylene glycol. Biotechnol. Bioprocess Eng. 12, 390–398 (2007). https://doi.org/10.1007/BF02931061
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931061