Summary
1. Expression of the apamin-sensitive K+ channel (SK+) in rat skeletal muscle is neurally regulated. The regulatory effect of the nerve over the expression of some muscle ion channels has been attributed to the electrical activity triggered by the nerve and/or to a trophic effect of some molecules transported from the soma to the axonal endings.
2. SK+ channels apparently are involved in myotonic dystrophy (MD), therefore understanding the factors that regulate their expression may ultimately have important clinical relevance.
3. To establish if axoplasmic transport is involved in this process, we used two experimental approaches in adult rats: (a) Both sciatic nerves were severed, leaving a short or a long nerve stump attached to the anterior tibialis (AT). (b) Colchicine or vinblastine (VBL), two axonal transport blockers of different potencies, was applied on one leg to the sciatic nerve. To determine whether electrical activity affects the expression of SK+ channels, denervated AT were directly stimulated. The corresponding contralateral muscles were used as controls.
4. With these experimental conditions we measured (a) apamin binding to muscle membranes, (b) muscle contractile characteristics, and (c) electromyographic activity.
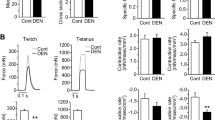
5. In the short- and long-nerve stump experiments, 5 days after denervation125I-apamin binding to AT membranes was 2.0 times higher in the short-stump side. This difference disappeared at longer times. The delayed expression of SK+ channels in the muscle left with a longer nerve stump can be attributed to the extra axoplasm contained in the longer stump, which maintains a normally repressive signal for a longer period of time. Ten to 15 days after application of axonal transport blockers we found that the muscle half-relaxation time increased in the drug-treated side and apamin partially reverted the prolonged relaxation. Myotonic-like discharges specifically blockable by apamin were always present in the drug-treated leg.125I-Apamin binding, which is undetectable in a microsomal preparation from hind leg control muscles, was increased in the drug-treated preparations. Apamin binding to denervated and stimulated AT muscles was lower than in the contralateral unstimulated muscles [3.3±1.0 vs 6.8±0.8 (n=4) fmol/mg protein].
6. Our results demonstrate that electrical activity and axoplasmic transport are involved in the control of expression of SK+ in rat skeletal muscle. However, the increased expression of this channel induces myotonic-like characteristics that are reversed by apamin. This myotonic activity could be a model for MD.
Similar content being viewed by others
References
Adrian, R. H., and Bryant, S. H. (1974). On the repetitive discharge in myotonic muscle fibers.J. Physiol. 240505–515.
Barchi, R. L., and Furman, R. E. (1992). Pathophysiology of myotonia and periodic paralysis. In Asbury, A. K., McKhann, G. M., and McDonald, W. J. (Eds.);Diseases of the Nervous System; Clinical Neurobiology, W. B. Saunders, Philadelphia, Vol. 1, pp. 146–163.
Behrens, M. I., and Vergara, C. (1992). Increase of apamin receptors in skeletal muscle induced by colchicine: Possible role in myotonia.Am. J. Physiol. 263C794-C802.
Behrens, M. I., Torrealba, G., Soza, M. A., Court, J., and Ramírez, B. U. (1983). Axonal transport dysfunction in dystrophia myotonica.Acta Neuropathol. 62157–158.
Behrens, M. I., Jalil, P., Serani, A., Vergara, F., and Alvarez, O. (1994). Possible role of apamin-sensitive K+ channels in myotonic dystrophy.Muscle Nerve 171264–1270.
Brook, J. D., McCurrach, M. E., Harley, H. G., Buckler, A. J., Church, D., Abuvatani, H., Hunter, K., Stanton, V. P., Thirion, J.-P., Hudson, T., Sohn, R., Zemelman, B., Snell, R. G., Rundle, S. A., Crow, S., Davies, J., Shelbourne, P., Buxton, J., Jones, C., Juronen, V., Johnson, K., Harper, P. S., Shaw, D. J., and Housman, D. E. (1992). Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat of the 3′ end of a transcript encoding a protein kinase family member.Cell 68 799–808.
Cannon, S. C. (1994). Slow sodium channel inactivation need not be disrupted in the pathogenesis of myotonia and periodic paralysis.Biophys. J. 66543–544.
Cannon, S. C., Brown, R. H., and Corey, D. P. (1993). Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels.Biophys J. 65270–288.
Chachine, M., George, A. L., Jr., Zhou, M., Ji, S., Sun, W., Barchi, R. L., and Horn, R. (1994). Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation.Neuron 12281–294.
Franke, C., Hatt, H., Iaizzo, P. A., and Lehmann-Horn, F. (1990). Characteristics of Na+ channels and Cl− conductance in resealed muscle fibre segments from patients with myotonic dystrophy.J. Physiol 425391–405.
Fu, Y.-H., Friedman, D. L., Richards, S., Pearlman, J. A., Gibbs, R. A., Pizzuti, A., Ashizawa, T., Perryman, M. B., Scarlato, G., Fenwick, R. G., Jr., and Caskey, C. T. (1993). Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy.Science 260235–238.
Hall, Z. W., and Sanes, J. R. (1993). Synaptic structure and development: The neuromuscular junction.Neuron 1099–121.
Hille, B. (1992).Ionic Channels of Excitable Membranes, Sinauer Associates, Sunderland, MA.
Huang, C. F., Neville, C. M., and Schmidt, J. (1993). Control of myogenic factor genes by the membrane depolarization/protein kinase C cascade in chick skeletal muscle.FEBS Lett. 31921–25.
Hugues, M., Romey, G., Duval, D., Vincent, J. P., and Lazdunski, M. (1982). Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: Voltage-clamp and biochemical characterization of the toxin receptor.Proc. Natl. Acad. Sci. 791308–1312.
Inestrosa, N. C., and Fernández, H. L. (1976). Muscle enzymatic changes induced by blockage of axoplasmic transport.J. Neurophysiol. 391236–1245.
Lomo, T., and Rosenthal, J. (1972). Control of the Ach sensitivity by muscle activity in the rat.J. Phys. 221493–513.
Luco, J. V., and Eyzaguirre C. (1955). Fibrillation and hypersensitivity to Ach in denervated muscle: Effect of length of degenerating nerve fibers.J. Neurophys. 1865–73.
Lupa, M. T., and Caldwell, J. H. (1994). Sodium channels aggregate at former synaptic sites in innervated and denervated regenerating muscles.J. Cell Biol. 124139–147.
McManus, O. (1991). Ca-activated K channels: Regulation by calcium. L.Bioenerg. Biomembr. 23561–576.
Mrak, R. E. (1985). InMuscle Membranes in Diseases of Muscle, CRC Press, Boca Raton, FL.
Ramírez, B. U. (1980). Neurotrophic regulation of muscle autolytic activity.Exp. Neurol. 67257–264.
Renaud, J. F., Desnuelle, C., Schmid-Antomarchi, H., Hugues, M., Serratrice, G., and Lazdunski, M. (1986). Expression of apamin receptors in muscles of patients with myotonic muscular dystrophy.Nature 319678–680.
Rudel, R., and Lehmann-Horn, F. (1985). Membrane changes in cells from myotonia patients.Physiol. Rev. 65310–356.
Rudel, R., Ruppersberg, J. P., and Spittelmeister, W. (1989). Abnormalities of the fast sodium current in myotonic dystrophy, recessive generalized myotonia, and adynamia episodica.Muscle Nerve 12281–287.
Sampson, S. R., and Alboim, S. V. (1993). Regulation of voltage-sensitive K-channels in cultured rat skeletal muscle.Soc. Neurosci. Abstr. 19706.
Schmid-Antomarchi, H., Renaud, J. F., Romey, G., Hugues, M., Schmid, A., and Lazdunski, M. (1985). The all-or-none response of innervation in expression of apamin receptor and apaminsensitive Ca2+-activated K+ channel in mammalian skeletal muscle.Proc. Natl. Acad. Sci. USA 822188–2191.
Schuetze, S. M., and Role L. (1987). Developmental regulation of nicotinic acetylcholine receptors.Annu. Rev. Neurosci. 10403–457.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ramírez, B.U., Behrens, M.I. & Vergara, C. Neural control of the expression of a Ca2+-activated K+ channel involved in the induction of myotonic-like characteristics. Cell Mol Neurobiol 16, 39–49 (1996). https://doi.org/10.1007/BF02578385
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02578385