Abstract
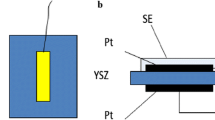
Stabilized zirconia-based electrochemical devices for which the sensing electrode was provided with a single-metal oxide were tested for NO and NO2 sensing properties at high temperature. Among the many single-metal oxides examined, WO3 was found to give the best sensing properties to NO and NO2 at 500–700°C. The EMF response of the WO3-attached device was linear to the logarithm of NO or NO2 concentration. The response and recovery kinetics were speedy. The device gave very small cross-sensitivities to H2, CO, CH4, CO2 and water vapor. The sensing mechanism involving mixed-potential was confirmed from the measurements of polarization curves.
Similar content being viewed by others
5. References
G. Sberveglieri, S. Groppelli and G. Coccoli, Sensors and Actuators15, 235 (1988).
M. Akiyama, J. Tamaki, N. Miura and N. Yamazoe, Chem. Lett.1991, 1611 (1991).
M. Akiyama, Z. Zhang, J. Tamaki, T. Harada, N. Miura and N. Yamazoe, Sensors and Actuators B13–14, 619 (1993).
J. Tamaki, T. Fujii, K. Fujimori, N. Miura and N. Yamazoe, Sensors and Actuators B24–25, 396 (1995).
S. Matsushima, D. Ikeda, K. Kobayashi and G. Okada, Chem. Lett.1992, 323 (1992).
Y. Sadaoka, N. Yamazoe and T. Seiyama, Denki Kagaku46, 597 (1978).
H. Mockert, D. Schmeisser and W. Göpel, Sensors and Actuators19, 159 (1989).
S. Dogo, J. P. Germain, C. Maleysson and A. Pauly, Thin Solid Films219, 251 (1992).
M. Gauthier and A. Chamberland, J. Electrochem. Soc.124, 1579 (1977).
G. Hötzel and W. Weppner, Solid State Ionics18/19, 1223 (1986).
S. Yao, Y. Shimizu, N. Miura and N. Yamazoe, Chem. Lett.1992, 587 (1992).
N. Miura, S. Yao, Y. Shimizu and N. Yamazoe, Solid State Ionics70/71, 572 (1994).
T. Harada, N. Miura and N. Yamazoe, Tech. Digest of 11th Sensor Symp., Kawasaki, Japan (1996) p. 241.
H. Kurosawa, Y. Yan, N. Miura and N. Yamazoe, Solid State Ionics79, 338 (1995).
N. Miura, M. Iio, G. Lu and N. Yamazoe, J. Electrochem. Soc.143, L241 (1996).
N. Miura, M. Iio, G. Lu and N. Yamazoe, Sensors and Actuators B35/36, 124 (1996).
N. Miura, M. Ono, K. Shimanoe and N. Yamazoe, Sensors and Actuators B49, 101 (1998).
G. Hötzel and W. Weppner, Sensors and Actuators B12, 449 (1987).
T. Ishihara, S. Sato and Y. Takita, Sensors and Actuators B24–25, 392 (1995).
N. Miura, G. Lu, N. Yamazoe, H. Kurosawa and M. Hasei, J. Electrochem. Soc.143, L33 (1996).
N. Miura, H. Kurosawa, M. Hasei, G. Lu and N. Yamazoe, Solid State Ionics86–88, 1069 (1996).
G. Lu, N. Miura and N. Yamazoe, J. Mat. Chem.7, 1445 (1997).
N. Miura, Y. Yan, G. Y. Lu and N. Yamazoe, Sensors and Actuators B34, 367 (1996).
G. Lu, N. Miura and N. Yamazoe, Sensors and Actuators B35/36, 130 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, G., Miura, N. & Yamazoe, N. High-temperature NO or NO2 sensor using stabilized zirconia and tungsten oxide electrode. Ionics 4, 16–24 (1998). https://doi.org/10.1007/BF02375775
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02375775