Abstract
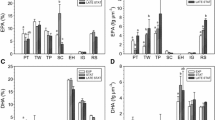
Three species of microalgae were grown in mass culture to investigate the influence of culture technique and growth phase on the production of 20:5(n−3) and 22:6(n−3). These polyunsaturated fatty acids (PUFA) are considered to be essential in many marine animals diets for high growth and survival rates. The species of microalgae examined wereNannochloropsis oculata, Pavlova lutheri andIsochrysis sp. (clone T.Iso). All batch cultures (logarithmic and stationary phase) and semi-continuous cultures (logarithmic phase) examined contained high levels of the long-chain (n−3) PUFA, but production could be maximised by harvesting at specific times and growth phases. Maximum cellular content (pg cell-1) of long-chain PUFA was found in logarithmic phase batch cultures ofN. oculata and in stationary phase cultures ofP. lutheri. The cellular content of PUFA in cultures ofIsochrysis sp. did not change significantly with culture technique or growth phase. Alternatively, stationary phase cultures of all three species showed increased proportions (%) and cellular contents of triacylglycerols, and saturated and monounsaturated fatty acids with correspondingly decreased proportions of polar lipids and most PUFA relative to logarithmic phase cultures. The exception was the proportion and cellular content of 22:6(n−3) inP. lutheri which increased with triacylglycerol content. The mass of long-chain (n−3) PUFA per volume of culture was significantly higher in stationary phase cultures due to the higher cell counts per volume. These findings indicate that the opportunity exists to maximise PUFA production by microalgae with the potential to improve animal growth and reduce production costs in mariculture operations and may be of use in the large scale culture and harvesting of microalgae for the biotechnology industry.
Similar content being viewed by others
References
Ackman RG, Tocher CS, McLachlan J (1968) Marine phytoplankter fatty acids. J. Fish. Res. Bd Canada 25: 1603–1620.
Ballantine JA, Lavis A, Morris RJ (1979) Sterols of the phytoplankton-effects of illumination and growth stage. Phytochemistry 18: 1459–1466.
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profiles of selected species of microalgae with emphasis on lipids. J. Phycol. 21: 72–81.
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 912–917.
Dunstan GA, Volkman JK, Jeffrey SW, Barrett SM (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae 2. Lipid classes and fatty acids. J. exp. mar. Biol. Ecol. 161: 115–134.
Emdadi D, Berland B (1989) Variation in lipid class composition during batch growth ofNannochloropsis salina andPavlova lutheri. Mar. Chem. 26: 215–225.
Enright CT, Newkirk GF, Craigie JS, Castell JD (1986) Evaluation of phytoplankton as diets for juvenileOstrea edulis L. J. exp. mar. Biol. Ecol. 96: 1–13.
Graske J, van der Molen M (1992) Effects of nutrient concentration on the growth of TahitianIsochrysis. Austasia Aquaculture 6: 43–47.
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. appl. Phycol. 2: 45–56.
Helm MM (1977) Mixed algal feeding ofOstrea edulis larvae withIsochrysis galbana andTetraselmis suecica. J. mar. biol. Ass. U.K. 57: 1019–1029.
Helm MM, Laing I (1987) Preliminary observations on the nutritional value of TahitiIsochrysis to bivalve larvae. Aquaculture 62: 281–288.
Hodgson PA, Henderson RJ, Sargent JR, Leftley JW (1991) Patterns of variation in the lipid class and fatty acid composition ofNannochloropsis oculata (Eustigmatophyceae) during batch culture I. The growth cycle. J. appl. Phycol. 3: 169–181.
Jeffrey SW (1980) Cultivating unicellular marine plants. In: CSIRO Division of Fisheries and Oceanography, Annual report (1977–1979), 22–43.
Jones DA, Kanazawa A, Ono K (1979) Studies on the nutritional requirements of the larval stages ofPenaeus japonicus using microencapsulated diets. Mar. Biol. 54: 261–267.
Kanazawa A, Teshima S, Imai K (1980) Biosynthesis of fatty acids inTilapia zillii and the puffer fish. Mem. Fac. Fish. Kagoshima Univ. 29: 313–318.
Kanazawa A, Teshima S, Kazuo O (1979) Relationship between essential fatty acid requirements of aquatic animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp. Biochem. Physiol. 63B: 295–298.
Langdon CJ, Waldock MJ (1981) The effect of algal and artificial diets on the growth and fatty acid composition ofCrassostrea gigas spat. J. mar. biol. Assoc. U.K. 61: 431–448.
Lewis TE, Garland CD, O'Brien TD, Fraser MI, Tong PA, Ward C, Dix TG, McMeekin TA (1988) The use of 0.2-µm membrane-filtered seawater for improved control of bacterial levels in microalgal cultures fed to larval Pacific oysters (Crassostrea gigas). Aquaculture 69: 241–251.
Lombardi AT, Wangersky PJ (1991) Influence of phosphorus and silicon on lipid class production by the marine diatomChaetoceros gracilis grown in turbidostat cage cultures. Mar. Ecol. Prog. Ser. 77: 39–47.
López Alonso D, Molina Grima E, Sánchez Pérez E, García Sánchez JL, García Camacho F (1992) Isolation of clones ofIsochrysis galbana rich in eicosapentaenoic acid. Aquaculture 102: 363–371.
Maruyama I, Nakamura T, Matsubayashi T, Ando Y, Maeda T (1986) Identification of the alga known as ‘marineChlorella’ as a member of the Eustigmatophyceae. Jap. J. Phycol. 34: 319–325.
Morris RJ, Sargent JR (1973) Studies on the lipid metabolism of some oceanic crustaceans. Mar. Biol. 22: 77–83.
Parrish CC, Wangersky PJ (1990) Growth and lipid class composition of the marine diatom,Chaetoceros gracilis, in laboratory and mass culture turbidostats. J. Plankton Res. 12: 1011–1021.
Piorreck M, Baasch K, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue green algae under different nitrogen regimes. Phytochemistry 23: 207–216.
Roessler PG (1990) Environmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J. Phycol. 26: 393–399.
Seto A, Wang HL, Hesseltine CW (1984) Culture conditions affect eicosapentaenoic acid content ofChlorella minutissima. J. am. Oil Chem. Soc. 61: 892–894.
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and lightdark cycles. J. Phycol. 17: 374–384.
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the greena algaNannochloropsis sp. QII under different nitrogen regimes. J. Phycol. 23: 289–296.
Sukenik A, Carmeli Y (1990) Lipid synthesis and fatty acid composition inNannochloropsis sp. (Eustigmatophyceae) grown in a light-dark cycle. J. Phycol. 26: 463–469.
Sukenik A, Wahnon R (1991) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. I.Isochrysis galbana. Aquaculture 97: 61–72.
Thompson PA, Harrison PJ, Whyte JNC (1990) Influence of irradiance on the fatty acid composition of phytoplankton. J. Phycol. 26: 278–288.
Volkman JK, Dunstan GA, Jeffrey SW, Kearney PS (1991) Fatty acids from microalgae of the genusPavlova. Phytochemistry 30: 1855–1859.
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. exp. mar. Biol. Ecol. 128: 219–240.
Yongmanitchai W, Ward OP (1989) Omega-3 fatty acids: alternative sources of production. Process Biochem. August: 117–125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dunstan, G.A., Volkman, J.K., Barrett, S.M. et al. Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J Appl Phycol 5, 71–83 (1993). https://doi.org/10.1007/BF02182424
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02182424