Abstract
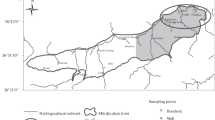
A geochemical study of groundwater of the pampa in the province of Córdoba, Argentina, was performed; the area covered approximately 10,000 km2.
Physical-chemical parameters, dissolved solids, and seven trace elements were determined in 60 selected water samples. Systematic and accurate measurements of arsenic, flourine, and vanadium were performed for the first time. Three trace element contaminants not reported earlier were found: an important one, selenium, and two others of less known effects, uranium and molybdenum.
Eighty-four percent of the water analyzed showed arsenic contents over 0.05 mg/L, maximum contaminant level established by the U.S. Environmental Protection Agency (1982). The frequency distribution of trace elements was analyzed, and its fit to the lognormal distribution was proved by means of the Pearson and Kolmogorov-Smirnov test; the geographic distribution of the seven trace elements was mapped and its correlation with the anion-cation composition of the water was studied.
The maximum arsenic, fluorine, vanadium, and uranium contents were found in the western part of the area under study, in waters containing dominant alkali metals in the cation composition. Maximum selenium and antimony contents were found in the eastern part of the area, while molybdenum distribution does not show any relationship with the other two groups. In addition, the geographic distribution of the trace elements seems to be related to the subsurface structure, which has been inferred using interactive digital analysis of Landsat imagery. The movements of the subsoil have disturbed surface and subsurface drainage influencing the water salinity and trace element contents.
In order to investigate the origin of the contamination, 54 loess samples were collected in wells at depths ranging from the surface down to the water table. This loess, which has a high proportion of volcanic components, mainly rhyolitic glass, exhibits a chemical composition corresponding to that of a dacite.
The loess and the volcanic glass show anomalous contents of all contaminant trace elements, mainly arsenic and selenium. For this reason loess is considered the most important contamination source in the groundwater under study.
Similar content being viewed by others
References Cited
Astolfi, E. A. N., A. Maccagno, J. C. García Fernández, R. Vaccaro, and R. Stimola, 1981, Relation between arsenic in drinking water and skin cancer: Biolog. Trace Element Res., v. 3, p. 133–143.
Bertini, L. M., and I. M. Cohen, 1984, Determination of arsenic, antimony and selenium in water by neutron activation and coprecipitation with bismuth sulphide: Fifth Internat. Conf. on Nuclear Methods in Environment and Energy Research, Mayaguez, Puerto Rico; Proceedings CONF-840408 (PE 84017348), p. 340–347.
Brown, E., M. W. Skougstad, and M.J. Fishman, 1970, Methods for collection and analysis of water samples for dissolved minerals and gases: U.S. Geol. Survey, Techniques of Water-Resources Investigations, book 5, chapter A 1, 160 p.
Buraczyński, J., 1979, Caractéristiques lithologiques des loess d'Achenheim (près de Strasbourg, France): Acad. Sci. Hungaricae Acta Geol., v. 22, p. 229–253.
Castellanos, A., 1959, Posibles desplazamientos, en el pasado, de las redes potamográficas en la llanura cordobesa: Boletín de Estudios Geográficos, no. 19, Inst. Geografía, Univ. Nac. de Córdoba, p. 29–63.
Church, B. N., 1975, Quantitative classification and chemical comparison of common volcanic rocks: Geol. Soc. America Bull., v. 86, p. 257–263.
Ebens, R. J., and J. J. Connor, 1980, Geochemistry of loess and carbonate residuum: U.S. Geol. Survey Prof. Paper 954-G, 32 p.
Ferpozzi, L. H., 1985, Geoquímica del arsénico y de otros elementos asociados en sedimentos limo-loessicos del sudeste de la provincia de Córdoba. III—Geoquímica de los sedimentos: Tech. Int. Rep., CONICET, 55 p.
Fisher, R. V., and H. U. Schmincke, 1984, Pyroclastic rocks: New York, Springer-Verlag, 528 p.
Folk, R. L., and W. C. Ward, 1957, Brazos River bar: a study in the significance of grain size parameters: Jour. Sed. Petrology, v. 27, p. 3–26.
Frenguelli, J., 1925, Loess y limos pampeanos: Anales Soc. Arg. Estudios Geográficos Gaea, v. 1, p. 1–88.
Heller-Kallai, L., and I. Rozenson, 1981, The use of Mössbauer spectroscopy of iron in clay mineralogy: Phys. Chem. Minerals, v. 7, p. 223–238.
IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Man, 1975: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man, v. 9, p. 245–260.
Kurkjian, C. R., and E. A. Sigety, 1968, Coordination of Fe3+ in glass: Phys. Chem. Glasses, v. 9, p. 73–83.
Loughnan, F. C., 1969, Chemical weathering of the silicate minerals: New York, Elsevier, 154 p.
Mysen, B. O., D. Virgo, and F. A. Seifert, 1984, Redox equilibria of iron in alkaline earth silicate melts: relationship between melt structure, oxigen fugacity, temperature and properties of iron-bearing silicate liquids: Am. Mineral., v. 69, p. 834–847.
Nicolli, H. B., 1982, Técnica de muestreo de aguas para el análisis de oligoelementos por activación: Tech. Int. Rep. C.N.I.E., G.E. 82/01, 8 p.
Nicolli, H. B., and M. A. Gamba, 1979, Guía para el muestreo geoquímico de aguas y salmueras: Pub. C.N.I.E., G.E. 79/01, 23 p.
Nicolli, H. B., T. E. O'Connor, J. M. Suriano, M. M. L. Koukharsky, M. A. Gómez Peral, L. M. Bertini, I. M. Cohen, L. I. Corradi, O. A. Baleani, and E. G. Abril, 1985, Geoquímica del arsénico y de otros oligoelementos en aguas subterráneas de la llanura sudoriental de la provincia de Córdoba: Miscelánea no. 71, Acad. Nac. Ciencias, Córdoba, 112 p.
Onishi, H., and E. B. Sandell, 1955, Geochemistry of arsenic: Geochim. Cosmochim. Acta, v. 7, p. 1–33.
Sarna-Wojcicki, A. M., H. R. Bowman, and P. C. Russell, 1979, Chemical correlation of some late Cenozoic tuffs of northern and central California by neutron-activation analysis of glass and comparison with X-ray fluorescence analysis: U.S. Geol. Survey Prof. Paper 1147, 15 p.
Sarna-Wojcicki, A. M., H. R. Bowman, C. E. Meyer, P. C. Russell, M. J. Woodward, G. McCoy, J. J. Rowe, Jr., P. A. Baedecker, F. Asaro, and H. Michael, 1984, Chemical analysis, correlations, and ages of upper Pliocene and Pleistocene ash layers of east-central and southern California: U.S. Geol. Survey Prof. Paper 1293, 40 p.
Taylor, S. R., 1964, Abundances of chemical elements in the continental crust: a new table: Geochim. Cosmochim. Acta, v. 28, p. 1273–1285.
Taylor, S. R., S. M. McLennan, and M. T. McCulloch, 1983, Geochemistry of loess, continental crustal composition and crustal model ages: Geochim. Cosmochim. Acta, v. 47, p. 1897–1905.
Teruggi, M. E., 1957, The nature and origin of Argentine loess: Jour. Sed. Petrology, v. 27, p. 322–332.
U.S. Environmental Protection Agency, 1982, Maximum contaminant levels (subpart B of part 141, National interim primary drinking-water regulations): U.S. Code of Federal Regulations, Title 40, Part 100 to 149, revised as of July 1, 1982, p. 315–318.
U.S. Geological Survey, 1979, Methods for determination of inorganic substances in water and fluvial sediments.In M. W. Skougstad, M. J. Fishman, L. C. Friedman, D. E. Erdmann, and S. S. Duncan, Eds, Techniques of Water-Resources Investigations of the United States Geological Survey, book 5, chapter A 1, 626 p.
U.S. Public Health Service, 1962, Drinking water standards, 1962: U.S. Public Health Service Pub. 956, 61 p.
Vázquez, J. B., 1979, Suelos. In: J. B. Vázquez, R. A. Miatello, and M. E. Roqué (Directors), Geografía de la Provincia de Córdoba, Córdoba, Fundación Banco Prov. de Córdoba, p. 435–458.
Wood, W. W., 1976, Guidelines for collection and field analysis of groundwater samples for selected unstable constituents: U.S. Geol. Survey Techniques of Water-Resources Investigations, book 1, chapter D 2, 24 p.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicolli, H.B., Suriano, J.M., Gomez Peral, M.A. et al. Groundwater contamination with arsenic and other trace elements in an area of the pampa, province of Córdoba, Argentina. Environ. Geol. Water Sci 14, 3–16 (1989). https://doi.org/10.1007/BF01740581
Issue Date:
DOI: https://doi.org/10.1007/BF01740581