Summary
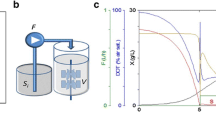
Two kinds of fed batch fermentation processes were compared at a 10-liter scale to examine their effect on recombinant human insulin-like growth factor (IGF-1) gene expression inEscherichia coli. The difference between the two processes was the feed medium composition and whether the process used a single or dual feed during the course of the fermentation. In the dual feed system, organic nitrogen was delivered at a higher rate (50 g/h) than in the single feed system (5 g/h). The dual feed process resulted in a significant increase in IGF-1 yield. 30 mg IGF-1/g dry cell weight was synthesized in the dual feed system compared to 3 mg IGF-1/g dry cell weight obtained in the single feed system. The presence of high levels of organic nitrogen during the induction period may enhance IGF-1 synthesis by protecting the IGF-1 from proteolytic degradation. The IGF-1 yield decreased to 17 mg/g dry cell weight when the glucose supply was decreased from 17 g/h to 8 g/h during the induction period; however, an increase in glucose supply from 17 g/h to 50 g/h during the induction period did not enhance the IGF-1 synthesis. Thus, the enhancement of IGF-1 gene expression in the dual feed process was mainly dependent on a high level of organic nitrogen and an appropriate level of glucose in the medium during the induction period.
Similar content being viewed by others
References
Alton, K., Y. Stabinksy, R. Richards, B. Ferguson, L. Goldstein, B. Altrock, L. Miller and N. Stebbing. 1983. Production, characterization and biological effect of recombinant DNA derived human IFN-α and IFN-γ analogs. In: The Biology of the Interferon System (De Maeyer, E. and Schellekens, H., eds.), pp. 119–127, Elsevier Science Publishers, Amsterdam.
Buell, G., M.F. Schulz, G. Selzer, A. Chollet, N. Rao Movva, D. Semon, S. Escanez and E. Kawashima. 1985. Optimizing the expression inE. coli of a synthetic gene encoding somatomedin-C (IGF-1). Nucl. Acids Res. 13: 1923–1938.
Clemmons, D.R. and J.J. Van Wyk. 1981. Somatomedin: physiological control and effect on cell proliferation. In: Handbook of Experimental Pharmacology, Vol. 57 (Basega, R., ed.), pp. 161–208, Springer-Verlag, Berlin.
Fieschko, J. and T. Ritch. 1986. Production of human alpha consensus interferon in recombinantEscherichia coli. Chem. Eng. Commun. 45: 229–240.
Goff, S.A., L.P. Casson and A.L. Goldberg. 1984. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon genes inEscherichia coli. Proc. Natl. Acad. Sci. USA 81: 6647–6651.
Grantham, R., C. Goutier, M. Gouy, M. Jacobzone and R. Mercier. 1981. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucl. Acids. Res. 9: r43-r74.
Klapper, D.G., M.E. Svoboda and J.J. Van Wyk. 1983. Sequence analysis of somatomedin C: Confirmation of identity with insulin-like growth factor I. Endocrinology 112: 2215–2217.
Mandelstam, J. 1960. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol. Rev. 24: 289–308
Neidhardt, F.C. and R.A. Van Bogelen. 1981. Positive regulatory gene for temperature controlled proteins inEscherichia coli. Biochem. Biophys. Res. Commun. 100: 894–900.
Neidhardt, F.C., R.A. Van Bogelen and E.T. Lou. 1983. Molecular cloning and expression of a gene that controls the high temperature regulation ofEscherichia coli. J. Bacteriol. 153: 597–603.
Peters, M.A., E.P. Lau, D.L. Snitman, J.J. Van Wyk, L.E. Underwood, W.E. Russel and M.E. Svoboda. 1985. Expression of a biologically active analogue of somatomedin-C/in-sulin-like growth factor I. Gene 35: 83–89.
Phillips, T.A., R.A. Van Bogelen and F.C. Neidhardt. 1984. Lon gene product ofEscherichia coli is a heat shock protein. J. Bacteriol. 159: 283–287.
Rinderknecht, E. and R.E. Humbel. 1978. The amino acid sequence of human insulin-like growth factor l and its structural homology with pro-insulin. J. Biol. Chem. 253: 1769–1776.
Schoenle, E., J. Zapf, R.E. Humbel and E.R. Froesch. 1982. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature 296: 252–253.
Van Wyk, J.J., L.E. Underwood, A.J. D'Ercole, D.R. Clemmons, W.J. Pledger, W.R. Wharton and E.B. Leof. 1981. Role of somatomedin in cellular proliferation. In: Biology of Normal Human Growth (Ritzen, E.B., ed.), pp. 223–239, Raven Press, New York.
Yamamori, T. and T. Yura, 1982. Genetic control of heat shock protein synthesis and its bearing on growth and thermal resistance inEscherichia coli re-12. Proc. Natl. Acad. Sci. USA 79: 860–864.
Zapf, J., E.R. Froesch and R.E. Humbel. 1981. The inslinlike growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr. Topics Cell. Reg. 19: 257–309.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsai, L.B., Mann, M., Morris, F. et al. The effect of organic nitrogen and glucose on the production of recombinant human insulin-like growth factor in high cell densityEscherichia coli fermentations. Journal of Industrial Microbiology 2, 181–186 (1987). https://doi.org/10.1007/BF01569426
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569426