Abstract
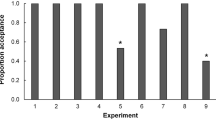
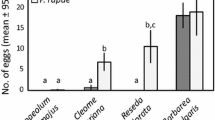
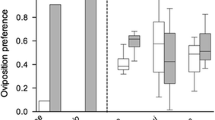
Numerous studies have documented the influence of environmental factors such as host plant species and host quality on the oviposition behavior of female insects. This paper shows that an internal physiological factor, the number of mature eggs a female carries (egg load), correlates with host selectivity and clutch size in unmanipulated natural populations of the pipevine swallowtail butterfly, Battus philenor.In addition, search intensity and host selectivity differed among females whose egg loads were manipulated experimentally before they were released and followed in the field. Females with many eggs searched more intensely for hosts and were less selective when they encountered them.
Similar content being viewed by others
References
Barker, J. F., and Herman, W. S. (1976). Effect of photoperiod and temperature on reproduction of the monarch butterfly,Danaus plexippus.J. Insect Physiol. 22: 1565–1568.
Carter, C. S. (1974).Hormones and Sexual Behavior, Dowden, Hutchinson & Ross, Stroudsburg, Pa.
Charnov, E. L., and Skinner, S. W. (1988). Clutch size in parasitoids: The egg production rate as a constraint.Evol. Ecol. 2: 167–174.
Chew, F. S. (1975). Coevolution of pierid butterflies and their cruciferous foodplants. I. The relative quality of available resources.Oecologia 20: 117–128.
Chew, F. S. (1977). Coevolution of pierid butterflies and their cruciferous foodplants. II. The distribution of eggs on potential foodplants.Evolution 31: 568–579.
Courtney, S. P. (1981). Coevolution of pierid butterflies and their curciferous foodplants. III.Anthocaris cardamines (L.) survival, development and oviposition on different hostplants.Oecologia (Berl.) 51: 91–96.
Courtney, S. P. (1982). Hostplant apparancy andAnthocharis cardamines oviposition.Oecologia (Berl.) 52: 258–265.
Courtney, S. P. (1986). The ecology of pierid butterflies: dynamics and interactions.Adv. Eco. Res. 15: 51–131.
David, W. A. L., and Gardiner, B. O. C. (1962). Oviposition and the hatching of the eggs ofPieris brassicae (L.) in a laboratory culture.Bull. Entomol. Res. 53: 91–109.
Dunlap-Pianka, H. L. (1979). Ovarian dynamics inHeliconius butterflies: Correlations among daily oviposition rates, egg weights, and quantitative aspects of oogenesis.tJ. Insect Physiol. 25: 741–749.
Edwards, P. J., and Wratten, S. D. (1982). Wound-induced changes in palatability in birch (Betula pubescens Ehrh ssp. Pubescens).Am. Nat. 120: 816–818.
Ehrlich, A. H., and Ehrlich, P. R. (1978). Reproductive strategies in the butterflies. I. mating frequency, plugging, and egg number.J. Kans. Entomol. Soc. 51(4): 66–697.
Farner, D. S., King, J. R., and Parkes, K. C. (eds.) (1971–1983).Avian Biology, Vols. I, III, V, VI, and VII, Academic Press, New York.
Gilbert, L. E., and Singer, M. C. (1975). Butterfly ecology.Annu. Rev. Ecol. Syst. 6: 365–397.
Gossard, T. W., and Jones, R. E. (1977). The effects of age and weather on egg-laying inPieris rapae L.J. Appl. Ecol. 14: 65–71.
Grossmueller, D. W., and Lederhouse, R. C. (1985). Oviposition site selection: An aid to rapid growth and development in the tiger swallowtail butterfly.Papilio glaucus.Oecologia 66: 68–73.
Holling, C. S. (1966). The functional response of invertebrate predators to prey density.Mem. Entomol Soc. Can. 48: 3–86.
Iwasa, Y., Odendaal, F. J., Murphy, D. D., Ehrlich, P. R., and Launer, A. L. (1983). Emergence patterns in male butterflies: A hypothesis and a test.Theor. Pop. Biol. 23: 363–379.
Iwasa, Y., Suzuki, Y., and Matsuda, H. (1984). Theory of oviposition strategy of parasitoids. I. Effect of mortality and limited egg number.Theor. Pop. Biol. 26: 205–227.
Jones, P. M. (1977a). Search behavior: A study of three caterpillar species.Behaviour 60: 237–259.
Jones, P. M. (1977b). Movement patterns and egg distributions in cabbage butterflies.J. Anim. Ecol. 46: 195–212.
Klomp, H., and Teerink, B. J. (1962). Host selection and number of eggs per oviposition in the egg-parasiteTrichogramma embryophagum Hbg.Nature 195: 1020–1021.
Lofts, B. (1974). Reproduction. In Lofts, B. (ed.),Physiology of the Amphibia, Vol. II, Academic Press, New York/London, pp. 107–218.
Ma, W. G., and Schoonhoven, L. M. (1973). Tarsal contact chemosensory hairs of the large white butterflyPieris brassicae and their possible role in oviposition behavior.Entomol. Exp. Appl. 16: 343–357.
MacDonald, P. T., and McInnes, D. O. (1985).Ceratitis capiatat: Effect of host fruit size on the number of eggs per clutch.Entomol. Exp. Appl. 37: 207–211.
Mackay, D. A. (1985). Within-population variation in pre-alighting search behavior and host plant selection by ovipositingEuphydryas editha butterflies.Ecology 56(1): 142–151.
Nijhout, H. F. (1984). Abdominal stretch reception inDipetalogaster maximus (Hemiptera: Reduviidae).J. Insect Physiol. 30(8): 629–633.
Odendaal, F. J. (1989). Mature egg number influences the behavior of femaleBattus philenor butterflies.J. Insect Behav. 2: 15–25.
Odendaal, F. J., Rausher, M. D., Benrey, B., and Nunez-Farfan, J. (1987). Predation byAnolis lizards onBattus philenor raises questions about butterfly mimicry systems.J. Lep. Soc. 41(3): 141–144.
Papaj, D. R. (1986). Shifts in foraging behavior by aBattus philenor population: Field evidence for switching by individual butterflies.Behav. Ecol. Sociobiol. 19: 31–39.
Papaj, D. R., and Rausher, M. D. (1983). Individual variation in host location by phytophagous insects. In Ahmad, S. (ed.),Herbivorous Insects: Host-Seeking Behavior and Mechanisms, Academic Press, New York.
Parker, G. A., and Courtney, S. P. (1984). Models of clutch size in insect oviposition.Theor. Pop. Biol. 26: 27–48.
Pilson, D., and Rausher, M. D. (1988). Clutch size adjustment by a swallowtail butterfly.Nature 333: 361–363.
Pitcher, T. J. (ed.) (1986).The Behavior of Teleost Fishes, Johns Hopkins University Press, Baltimore, Md.
Pouzat, J. (1978). Host plant chemosensory influence on oogenesis in the bean weavil,Acanthoscelides obtectus.Entomol. Exp. Appl. 24: 601–608.
Rankin, J. C., Pitcher, T. J., and Duggan, R. T. (1983).Control Processes in Fish Physiology, Wiley, New York.
Rankin, M. A. (1974). The hormonal control of flight in the milkweed bugOncopeltis fasciatus. In Barton Browne, L. (ed.),Experimental Analysis of Insect Behaviour, Springer-Verlag, New York, pp. 317–328.
Rausher, M. D. (1978). Search image for leaf shape in a butterfly.Science 200: 1071–1073.
Rausher, M. D. (1979a.) Egg recognition: Its advantage to a butterfly.Anim. Behav. 27: 1034–1040.
Rausher, M. D. (1979b). Larval habitat suitability and oviposition preference in three related butterflies.Ecology 60: 503–511.
Rausher, M. D. (1980). Host abundance, juvenile survival, and oviposition preference inBattus philenor.Evolution 34: 342–355.
Rausher, M. D. (1986). Competition, frequency-dependent selection, and diapause inBattus philenor butterflies.Fla. Entomol. 69(1): 63–78.
Rausher, M. D., and Odendaal, F. J. (1987). Switching and the pattern of host use byBattus philenor.Ecology 68(4): 869–877.
Rausher, M. D., and Papaj, D. R. (1983). Host plant selection byBattus philenor butterflies: Evidence for individual differences in foraging behaviour.Anim. Behav. 31: 341–347.
Root, R. B., and Kareiva., P. M., (1984). The search for resources by cabbage butterflies (Pieris rapae): Ecological consequences and adaptive significance of Markovian movements in a patchy environment.Ecology 65: 147–165.
Rothschild, M., and Schoonhoven, L. M. (1977). Assessment of egg load byPieris brassicae (Lepidoptera, Pieridae).Nature 266: 352–355.
Schultz, J. C. (1983a). Habitat selection and foraging tactics of caterpillars in heterogeneous trees. In Dennos, R. F., and McClure, M. S. (eds.),Herbivores in Natural and Managed Systems, Academic Press, New York, pp. 61–91.
Schultz, J. C. (1983b). Impact of variable plant defensive chemistry of susceptibility of insects to natural enemies. In Hedin, A. (ed.),Plant Resistance to Insects, American Chemical Society Symposium No. 208, Washington, D.C., pp. 37–54.
Shapiro, A. M. (1980). Egg-load assessment and carryover diapause inAnthocaris (Pieridae).J. Lepid. Soc. 34: 307–315.
Skinner, S. W. (1985). Clutch size as an optimal foraging problem in insects.Behav. Ecol. Sociobiol. 17: 231–238.
Smiley, J. (1978). Plant chemistry and the evolution of host specificity: New evidence fromHeliconius andPassiflora.Science 201: 745–747.
Singer, M. C. (1971). Evolution of food plant preferences in the butterflyEuphydryas editha.Evolution 25: 383–389.
Singler, M. C. (1982). Quantification of host preference by manipulation of oviposition behavior in the butterflyEuphydryas editha.Oecologia (Berl.) 52: 224–229.
Singer, M. C. (1983). Determinants of multiple host use by a phytophagous insect population.Evolution 37: 389–403.
Singer, M. C. (1986). The definition and measurement of oviposition preference in plant-feeding insects. In Miller, J. (ed.),Insect-Plant Relations, Springer-Verlag. New York.
Stanton, M. L. (1979). The role of chemotactile stimuli in the oviposition preferences ofColias butterflies.Oecologia 39: 79–91.
Stanton, M. L. (1982). Searching in a patchy environment: Foodplant selection byColias philodice butterflies.Ecology 63: 839–853.
Stern, V. M., and Smith, R. F. (1960). Factors affecting egg production and oviposition in populations ofColias philodice eurytheme Biosduval (Lepidoptera: Pieridae).Hilgardia 29(10): 411–454.
Streng, D. R., and Harcombe, P. A. (1982). Why don't East Texas savannas grow up to a forest?Am. Midl. Nature. 108: 278–294.
Van Tienhoven, A. (1983).Reproductive Physiology of Vertebrates. 2nd ed., Cornell University press, Ithaca, N.Y.
Vogl, R. J. (1972). Fire in the Southeastern grasslands.Tall Timbers Fire Ecol. Conf. 12: 175–198.
Watt, W. B., Chew, F. S., Snyder, L. R. G., Watt, A. G., and Rothschild, D. E. (1977). Population structure of Pierid butterflies. I. Numbers and movements of some montaneColias species.Oecologia 27: 1–22.
Wiklund, C. (1975). The evolutionary relationship between adult oviposition preference and larval host range inPapilio machaon L. Oecologia (Berl.) 18: 185–197.
Williams, K. S. (1983). The coevolution ofEuphydryas chalcedona butterflies and their larval host plants. III. Oviposition behavior and host plant quality.Oecologia (Berl.) 56: 335–340.
Williams, K. S., and Gilbert, L. E. (1981). Insects as selective agents on plant vegetative morphology: Egg mimicry reduces egg laying by butterflies.Science 212: 467–469.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Odendaal, F.J., Rausher, M.D. Egg load influences search intensity, host selectivity, and clutch size inBattus philenor butterflies. J Insect Behav 3, 183–193 (1990). https://doi.org/10.1007/BF01417911
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01417911