Abstract
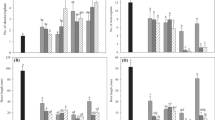
Axenic tissue cultures ofRuppia maritima L. were established and propagated clonally in vitro from terminal rhizome segments collected from Tampa Bay, Florida, USA. Cultures were maintained in a base medium consisting of synthetic seawater supplemented with half-strength Murashige and Skoog salts and 1% sucrose at pH 5.6. The effects of five cytokins [6-furfurylaminopurine (kinetin), 6-benzylaminopurine (BAP), 2-isopentyladenine (2iP), 6-(4-hydroxy-3-methyl-but-2-enylamino) purine (zeatin), andn-phenyl-n′-1,2,3-thidiazol-5′yl urea (thidiazuron)] and one auxin [napthalene acetic acid (NAA)] on explant growth and development were investigated. Cytokinin additions resulted in a 3- to 4-fold increase in nodal production, branching, and biomass ofR. maritima after 12 wk in culture. Cultures responded in a dose-dependent manner to 2iP but exhibited broad dose-response curves to kinetin, BAP, zeatin, and thidiazuron. NAA addition resulted in increased leaf and internodal lengths, but reduced the number of leaves per node and the rhizome biomass. The addition of NAA almost completely suppressed root growth in media without cytokinins and had an antagonistic effect on nodal production and branching in cytokinin-supplemented media.
Similar content being viewed by others
Literature cited
Abe, H., Uchiyama, M., Sato, R. (1972). Isolation and identification of native auxins in marine algae. Agric. biol. Chem. 36: 2259–2260
Ailstock, M. S. (1986). Clonal propagation ofPotamogeton pectinatus in axenic culture. In: Webb, F. J. (ed.) Proceedings of the 13th Annual Conference on Wetlands Restoration and Creation. Hillsborough Community College, Tampa, Florida, p. 11–17
Buggeln, R. G., Craigie, J. S. (1971). Evaluation of evidence for the presence of indole-3-acetic acid in marine algae. Planta 97: 173–178
Dawes, C. J. (1971). Indole-3-acetic acid in the green algal coenocyteCaulerpa prolifera (Chlorophyceae, Siphonales). Phycologia 10: 375–379
Durako, M. J. (1988a). Turtle grass (Thalassia testudinum Banks ex König) — a seagrass. In: Bajaj, Y. P. S. (ed.) Biotechnology in agriculture and forestry. Vol. 6. Crops II. Springer-Verlag, Berlin, Heidelberg, New York, p. 504–520
Durako, M. J. (1988b). Growth responses of light- and dark-cultivated axenicThalassia testudinum seedlings to organic carbon amendment and pH. EOS Trans., Am. geophys. Un. 69: p. 1115
Durako, M. J., Moffler, M. D. (1987). Nutritional studies of the submerged marine angiospermThalassia testudinum. I. Growth responses of axenic seedlings to nitrogen enrichment. Am. J. Bot. 74: 234–240
Forsberg, D. (1966). Sterile germination requirement of seed of some water plants. Physiologia Pl. 8: 128–137
Fries, L. (1977). Growth regulating effects of phenylacetic acid and p-hydroxyphenylacetic acid inFucus spiralis L. (Phaeophyceae, Fucales) in axenic culture. Phycologia 16: 451–456
Gerloff, G. C., Krombholz, P. H. (1966). Tissue analysis as a measure of nutrient availability for the growth of angiosperm aquatic plants. Limnol. Oceanogr. 11: 529–537
Grime, J. P. (1979). Plant strategies and vegetation processes. Wiley & Sons, New York
Gusev, M. V., Tambiev, A. H., Kirikova, N. N., Shelyastina, N. N., Aslanyan, R. R. (1987). Callus formation in seven species of agarophyte marine algae. Mar. Biol. 95: 593–597
Husband, B. C., Hickman, M. (1985). Growth and biomass allocation ofRuppia occidentalis in three lakes differing in salinity. Can. J. Bot. 63: 2004–2014
Kane, M. E., Sheehan, T. J., Ferwerda, F. (1988). In vitro growth of American lotus embryos. HortSci. 23: 611–613
Kelly, J. A., Fuss, C. M., Hall, J. R. (1971). The transplanting and survival of turtle grassThalassia testudinum, in Boca Ciega Bay, Florida. Fish. Bull. U.S. 69: 273–280
Kentzer, T., Synak, R., Burkiewicz, K., Barnas, A. (1980). Cytokinin-like activity in sea water andFucus vesiculosus L. Biologia Pl. 22: 218–225
Klaine, S. J. (1986). Influence of thidiazuron on propagule formation inHydrilla verticillata. J. aquat. Pl. Mgmt 24: 80–82
Kuo, J., McComb, A. J., Cambridge, M. L. (1981). Ultra-structure of the seagrass rhizosphere. New Phytol. 89: 139–143
Martin, J. T., Juniper, B. E. (1970). The cuticles of plants. Edward Arnold Press, London
Maruyama, A., Maeda, M., Simidu, U. (1986). Occurrence of plant hormone (cytokinin)-producing bacteria in the sea. J. appl. Bact. 61: 569–574
Maruyama, A., Maeda, M., Simidu, U. (1989). Microbial production of auxin indole-3-acetic acid in marine sediments. Mar. Ecol. Prog. Ser. 58: 69–75
Mishra, A. K., Kefford, N. P. (1969). Developmental studies on the coenocytic alga,Caulerpa sertularioides. J. Phycol. 5: 103–109
Moffler, M. D., Durako, M. J. (1984). Axenic culture ofThalassia testudinum Banks ex König (Hydrocharitaceae). Am. J. Bot. 71: 1455–1460
Mooney, P. A., van Staden, J. (1968). Algae and cytokinins. J. Pl. Physiol. 123: 1–21
Murashige, T. (1974). Plant propagation through tissue cultures. A. Rev. Pl. Physiol. 25: 135–166
Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Pl. 15: 473–497
Newell, S. Y., Fell, J. W. (1980). Mycoflora of turtle grass (Thalassia testudinum König) as recorded after seawater incubation. Botanica mar. 23: 265–275
Pedersen, M: (1968).Ectocarpus fasciculatus: marine brown alga requiring kinetin. Nature, Lond. 218: p. 776
Pedersen, M. (1973). Identification of a cytokinin, 6-(3-methyl-2-butenylamino) purine, in seawater and the effects of cytokinins on brown algae. Physiologia Pl. 28: 101–105
Pringsheim, E. G., Pringsheim, O. (1962). Axenic cultures ofUtricularia. Am. J. Bot. 49: 898–901
Schmidt, M. A., Hayasaka, S. S. (1985). Localization of a dinitrogen-fixingKlebsiella sp. isolated from root-rhizomes of the seagrassHalodule wrightii Aschers. Botanica mar. 27: 437–442
Seeliger, U., Cordazzo, C., Koch, E. W. (1984). Germination and algae-free laboratory culture of widgeon grass,Ruppia maritima. Estuaries 7: 176–178
Sieburth, J. M., Brooks, R. D., Gessner, R. V., Thomas, C. D., Tottle, J. L. (1974). Microbial colonization of marine plant surfaces as observed by scanning electron microscopy. In: Colwell, R. R., Morita, R. Y. (eds.) Effects of the ocean environment on microbial activities. University Park Press, Baltimore, p. 418–432
Smith, G. W., Hayasaka, S. S. (1982). Nitrogenase activity associated withHalodule wrightii roots. Appl. envirl Microbiol. 43: 1244–1248
Tay, S. A., MacLeod, J. K., Palni, L. M., Letham, D. S. (1985). Detection of cytokinins in a seaweed extract. Phytochem. 24: 2611–2614
Thomas, J. C., Katterman, F. R. (1986). Cytokinin activity induced by thidiazuron. Pl. Physiol. 81: 681–683
Thorpe, T. A., Patel, K. R. (1984). Clonal propagation: adventitious buds. In: Vasil, I. K. (ed.) Cell culture and somatic cell genetics of plants. Vol. 1. Academic Press, New York, p. 49–60
Thursby, G. W. (1984). Nutritional requirements of the submerged marine angiosperm,Ruppia maritima in algae-free culture. Mar. Ecol. Prog. Ser. 16: 45–50
Van Breedveld, J. (1975). Transplanting seagrasses with emphasis on the importance of substrate. Fla mar. Res. Publs 17: 1–26
Varner, J. E., Tuan-Hua Ho, D. (1976). Hormones. In: Bonner, J., Varner, J. E. (eds.) Plant biochemistry. Academic Press, New York, p. 713–770
Vasil, I. K., Vasil, V. (1980). Clonal propagation. In: Vasil, I. K. (ed.) Perspectives in plant cell and tissue culture. Academic Press, New York, p. 145–165
Verhoeven, J. T. A. (1979). The ecology ofRuppia-dominated communities in Western Europe. I. Distribution ofRuppia representatives in relation to their autecology. Aquat. Bot. 6: 197–268
Wetzel, R. G., McGregor, D. L. (1968). Axenic culture and nutritional studies of aquatic macrophytes. Am. Midl. Nat. 80: 52–64
Author information
Authors and Affiliations
Additional information
Communicated by J.M. Lawrence, Tampa
Rights and permissions
About this article
Cite this article
Koch, E.W., Durako, M.J. In vitro studies of the submerged angiospermRuppia maritima: Auxin and cytokinin effects on plant growth and development. Mar. Biol. 110, 1–6 (1991). https://doi.org/10.1007/BF01313085
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01313085