Abstract
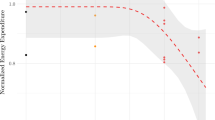
Behavioral and physiological responses of common grackles to dietary concentrations of dicrotophos, fenitrothion, fenthion, and methyl parathion suggest mortality was largely due to pesticide-induced anorexia. Mortality was dose related, though consumption of treated diets was reduced such that birds on different geometrically arranged concentrations of the same pesticide ingested about the same amount of toxicant. Grackles that died lost an average of 28 to 36% of their initial body weight; visible fat was absent and muscle tissue was reduced on the sternum. Mortality of birds exposed to dicrotophos increased between May and August, although chemical intake remained relatively constant, and was associated with a natural decrease in fat and flesh condition in response to increased ambient temperatures and post-nuptial molt. Food consumption in songbirds exposed to organophosphates may be reduced significantly up to 12 hr after exposure ceases because of an unknown effect of these chemicals on their feeding behavior, but not repellency. The results caution against using median lethal dietary concentrations for other than ranking chemicals based on their relative toxicity, particularly in establishing safe environmental levels, and suggest that anorexia and physiological condition may be important factors in mortality of wild birds exposed to organophosphates.
Similar content being viewed by others
References
Austin, O. L., Jr.: Families of Birds. New York: Golden Press (1971).
Bart, J.: Effects of acephate and Sevin® on forest birds. J. Wildl. Manage.43, 544 (1979).
Brust, R. A., S. Miyazaki, and G. C. Hodgson: Effect of Dursban® in the drinking water of chicks. J. Econ. Entomol.64, 1179(1971).
Dieter, M. P., and J. L. Ludke: Studies on combined effects of organophosphates and heavy metals in birds. I. Plasma and brain cholinesterase in coturnix quail fed methyl mercury and orally dosed with parathion. Bull. Environ. Contam. Toxicol.13, 257 (1975).
Duncan, D. B.: Multiple range and multiple F tests. Biometrics11, 1 (1955).
Ellman, G. L., K. D. Courtney, V. Andreas, Jr., and R. M. Featherstone: A new rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.7, 88 (1961).
Finley, R. B., Jr.: Adverse effects on birds of phosphamidon applied to a Montana forest. J. Wildl. Manage.29, 580 (1965).
Fowle, D. C: Effects of phosphamidon on forest birds in New Brunswick. Canadian Wildlife Service Report No.16, Ottawa, Canada (1972).
Heath, R. G., and L. F. Stickel: Protocol for testing the acute and relative toxicity of pesticides to penned birds. In The effects of pesticides on fish and wildlife. U.S. Fish and Wildlife Service Circular226, 18 (1965).
Hill, E. F.: Toxicity of selected mosquito larvicides to some common avian species. J. Wildl. Manage.35, 757 (1971).
—: Avoidance of lethal dietary concentrations of insecticide by house sparrows. J. Wildl. Manage.36, 635 (1972).
—: Cholinesterase activity in Japanese quail dusted with carbaryl. Lab. Anim. Sci.29, 349 (1979).
Hill, E. F., R. G. Heath, J. W. Spann, and J. D. Williams: Lethal dietary toxicities of environmental pollutants to birds. U.S. Fish and Wildlife Service Special Scientific Report—Wildlife191 (1975).
Keith, J. O., and M. S. Mulla: Relative toxicity of five organophosphorus mosquito larvicides to mallard ducks. J. Wildl. Manage.30, 553 (1966).
Kramer, C. Y.: Extension of multiple range tests to group means with unequal numbers of replication. Biometrics12, 307 (1956).
Litchfield, J. T., Jr., and F. Wilcoxon: A simplified method of evaluating dose-effect experiments. J. Pharmacol. and Exp. Therapeutics96, 99 (1949).
Ludke, J. L., E. F. Hill, and M. P. Dieter: Cholinesteratse (ChE) response and related mortality among birds fed ChE inhibitors. Arch. Environ. Contam. Toxicol.3, 1 (1975).
McLeod, J. M.: The effect of phosphamidon on bird populations in jack pine stands in Quebec. Can. Field Nat.81, 102 (1967).
Mehrotra, K. N., Y. P. Beri, S. S. Misra, and A. Phokela: Physiological effects of malathion on the house sparrow. Indian J. Exp. Biol.5, 219 (1967).
National Oceanic and Atmospheric Administration: Climatological data. Maryland and Delaware82 (1978).
Pearce, P. A.: Side effects of forest spraying in New Brunswick. N. Am. Wildl. Conf.36, 263 (1971).
Pillmore, R. E., E. L. Flickinger, and M. L. Richmond: Forest spraying of Zectran® and its safety to wildlife. J. Forestry69, 721 (1971).
Pope, G. G., and P. Ward: The effects of small applications of an organophosphorus poison, fenthion on the weaver-birdQuelea quelea. Pest. Sci.3, 197 (1972).
Rauen, V. H. M., H. Schriewer, B. Gebauer, M. Abu Tair, N. Ruther, and L. G. The: Multivariate analyse experimenteller Leberschadigungen: Normal parameter des Rattenserums. Arzneim-Forsch23, 108 (1973).
Salter, D. W.: Field and laboratory studies involving some of the effects of parathion on birds. M.S. thesis, University of Nevada, Reno (1966).
Seabloom, R. W., G. L. Pearson, L. W. Oring, and J. R. Reilly: An incident of fenthion mosquito control and subsequent avian mortality. J. Wildl. Diseases9, 18 (1973).
Snedecor, G. W., and W. G. Cochran: Statistical methods Ames: Iowa State University Press (1967).
Stevenson, H. M.: Florida region: Pesticides. Am. Birds26, 593 (1972).
Stickel, W. H.: Effects on wildlife of newer pesticides and other pollutants. Proc. Ann. Conf. West. Assoc. Game Fish Comm.53, 484 (1974).
Stone, W. B.: Poisoning of wild birds by organophosphate and carbamate pesticides. N.Y. Fish Game J.26, 37 (1979).
Tan, K. H.: An electrophoretic study of some hydrolases from fat-body and haemolymph of adult cave-roachPycnoscelus striatus (Kirby) using selective substrates and inhibitors. Comp. Biochem. Physiol.52C, 127 (1975).
U.S. Department of the Interior: Effects of Bidrin® on rangeland wildlife. In Wildlife research-problems, programs, progress 1967. Bureau of Sport Fisheries and Wildlife, Resource Publication74, 55 (1969).
Villeneuve, D. C., M. J. van Logten, E. M. den Tonkelaar, A. G. Rauws, R. Kroes, and G. J. van Esch: The combined effect of food restriction and parathion exposure in rats. Arch. Environ. Contam. Toxicol.7, 37 (1978).
Wolfson, A.: The role of the pituitary, fat deposition, and body weight in bird migration. Condor47, 95 (1945).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grue, C.E. Response of common grackles to dietary concentrations of four organophosphate pesticides. Arch. Environ. Contam. Toxicol. 11, 617–626 (1982). https://doi.org/10.1007/BF01056371
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01056371