Abstract
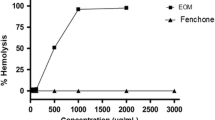
The genotoxic, cytotoxic and antitumor properties ofCommiphora molmol (oleo gum resin) were studied in normal and Ehrlich ascites carcinoma cell-bearing mice. In normal mice, the genotoxic and cytotoxic activity was evaluated on the bases of the frequency of micronuclei and the ratio of polychromatic to normochromatic cells in bone marrow, which was substantiated by the biochemical changes in hepatic cells. The antitumor activity ofC. molmol was evaluated from the total count and viability of Ehrlich ascites carcinoma cells and their nucleic acid, protein, malondialdehyde, and elemental concentrations in addition to observations on survival and the trend of changes in body weight. The tumors at the site of injection were evaluated for histopathological changes. Treatment withC. molmol (125–500 mg/kg) showed no clastogenicity but was found to be highly cytotoxic in normal mice. The results obtained in the Ehrlich ascites carcinoma cell-bearing mice revealed the cytotoxic and antitumor activity ofC. molmol which was found to be equivalent to those of the standard cytotoxic drug cyclophosphamide. On the basis of the nonmutagenic, antioxidative, and cytotoxic potential ofC. molmol as observed in the present study, its use in cancer therapy seems to be appropriate and further investigations are suggested.
Similar content being viewed by others
References
Tyler VE, Brady LR, Robbers JE (1981) Phammacognosy, 8th edn. Lea and Febiger, Philadelphia, pp 157–158
Evan WC (1989) Trease and Evan's pharmacognosy, 13th edn. Bailliere Tindall, London, pp 474–475
Ageel AM, Mossa JS, Tariq M, Al-Yahya MA, Al-Said MS (1987) Plants used in Saudi folk medicine. King Saud University Press, Riyadh, Saudi Arabia, p 121
Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS, Parmar NS (1985) Antiinflammtory activity ofCommiphora molmol. Agents Actions 17:381–382
Satyavati GV, Raghunathan K, Prasad DN, Rathore RS (1969)Commiphora mukul Engl. andTinospora cordifolia Willd. A study of the antiinflammatory activity. Rheumatism 4: 141–143
Brieskorn CH, Boble P (1983) Furanosesquiterpenes from the essential oil of myrrh. Phytochemistry 22: 1207–1211
Leung AY (1980) Encyclopedia of common natural ingredients used in food, drugs and cosmetics. John Wiley & Sons, New York, pp 241–242
Doskotch RW, Hufford CD, El-Feraly FS (1972) Antitumor agents. VI. Sesquiterpene lactones, tulipinolide and epitulipinolide fromLiriodendron tulipifera. J Org Chem 37: 2740–2744
Lien EJ, Wen YL (1985) Structure activity relationship analysis of anticancer Chinese drugs and related plants. Oriental Healing Arts Institute, USA, Taipei, Taiwan, pp 10–21
Cohen BI, Raicht RF (1981) Plant sterols: protective role in chemical carcinogenesis. In: Zedeck MS, Lipkin M (eds) Inhibition of tumor induction and development. Plenum, New York London, pp 189–201
Thompson DC, Constantin TD, Moldens P (1991) Metabolism and cytotoxicity of eugenol in isolated rat hepatocytes. Chem Biol Interact 77: 137–147
Hartwell JL (1982) Plants used against cancer. A survey. Quarterman, Lawrence, Massachusetts, pp 89–93
Schmid W (1975) The micronucleus test. Mutat Res 33: 9–15
Lowry OH, Rosebrough NS, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
Bregman AA (1983) Laboratory investigations and cell biology. John Wiley & Sons, New York, pp 51–60
Kaltenbach JP, Kaltenbach MH, Lyons WB (1958) Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res 15: 112–117
Fong KL, McCay PB, Poyer JL (1973) Evidence that peroxidation of lysosomal membrane is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem 248: 7792–7797
Whitehouse RC, Prasad AS, Rabbani PI, Cossack ZT (1982) Zinc in plasma, neutrophils, lymphocytes and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem 28: 475–480
Culling CFA (1974) Handbook of histopathological and histochemical techniques, 3rd edn. Butterworth, London, pp 73, 126, 159
Al-Bekairi AM, Qureshi S, Chaudhry MA, Shah AH (1991) Uric acid as an inhibitor of cyclophosphamide-induced micronuclei in mice. Mutat Res 262: 115–118
Oleinik AV (1986) Effect of cyclophosphane on lipid peroxidation. Vopr Onkol 31: 97–101
Oleinik AV (1986) Effect of cyclophosphane on bile formation and lipid peroxidation in the liver. Farmakol Toksikol 49: 51–54
Alldrick AJ, Lake BG, Rowland IR (1989) Modification of heterocyclic amine genotoxicity by dietary flavonoids. Mutagenesis 4: 365–370
Al-Bekairi AM, Qureshi S, Ahmed MM, Qazi NS, Khan ZA, Shah AH (1992) Effect ofCaralluma tuberculata on the cytological and biochemical changes induced by cyclophosphamide in mice. Food Chem Toxicol 30: 719–722
Buick RN (1984) The cellular basis of cancer chemotherapy. In: Remers WA (ed) Antineoplastic agents. John Wiley & Sons, Toronto, pp 1–40
Halliwell B, Gutteridge JMC (1987) Free radicals in biology and medicine. Clarendon, Oxford, pp 296–313
Borek C (1988) Oncogenes, hormones and free-radical processes in malignant transformation in vitro. Ann NY Acad Sci 551: 95–101
Taira A (1991) Active oxygen scavenger selected from conifaryl benzoate, eugenol, dehydrodiisoeugenol and isoeugenol. Nippon Kakai Tokkyo Koho, Tokyo, pp 3, 263, 481
Shah AH, Qureshi S, Tariq M, Ageel AM (1989) Toxicity studies on six plants used in the traditional Arab system of medicine. Phytother Res 3: 25–29
Berrigan MJ, Struck RF, Gurtoo HL (1987) Lipid peroxidation induced by cyclophosphamide. Cancer Biochem Biophys 9: 265–270
Patel JM (1987) Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology 45: 79–91
Koch KS, Leffert HL (1979) Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell 18: 153–163
Rozengurt E, Mendoza S (1980) Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann NY Acad Sci 339: 175–190
Smith JB, Rozengurt E (1978) Serum stimulates the Na+, K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci USA 75: 5560–5564
Villereal M (1981) Sodium fluxes in human fibroblasts: effects of serum, Ca2+ and amiloride. J Cell Physiol 107: 359–369
Sparks R, Pool TB, Smith NKR, Cameron IL (1983) Effects of Amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res 43: 73–77
Trump BF, Berezesky IK (1984) Role of sodium and calcium regulation in toxic cell injury. In: Mitchell JR, Horning MG (eds) Drug metabolism and drug toxicity. Raven, New York, pp 261–300
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qureshi, S., Al-Harbi, M.M., Ahmed, M.M. et al. Evaluation of the genotoxic, cytotoxic, and antitumor properties ofCommiphora molmol using normal and Ehrlich ascites carcinoma cell-bearing Swiss albino mice. Cancer Chemother. Pharmacol. 33, 130–138 (1993). https://doi.org/10.1007/BF00685330
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685330