Summary
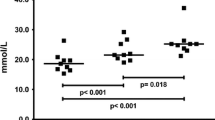
The content of anserine and carnosine in the lateral portion of the quadriceps femoris muscle of 50 healthy, human subjects has been studied. Anserine was undetectable in all muscle samples examined. Muscle carnosine values for the group conformed to a normal distribution with a mean (SD) value of 20.0 (4.7) mmol · kg−1 of dry muscle mass. The concentration of carnosine was significantly higher in the muscle of male subjects (21.3, 4.2 mmol · kg−1 dry mass) than in females of a similar age and training status (17.5, 4.8 mmol · kg−1 dry mass) (P< 0.005). The test-retest reliability of measures was determined on a subgroup of 17 subjects. No significant difference in mean carnosine concentration was found between the two trials [21.5 (4.0) and 22.0 (5.2) mmol · kg−1 dry muscle mass; P>0.05]. The importance of carnosine as a physicochemical buffer within human muscle was examined by calculating its buffering ability over the physiological pH range. From the range of carnosine concentrations observed (7.2–30.7 mmol · kg−1 dry muscle mass), it was estimated that the dipeptide could buffer between 2.4 and 10.1 mmol H+ · kg−1 dry mass over the physiological pH range 7.1–6.5, contributing, on average, approximately 7% to the total muscle buffering. This suggests that in humans, in contrast to many other species, carnosine is of only limited importance in preventing the reduction in pH observed during high intensity exercise.
Similar content being viewed by others
References
Avena RM, Bowen WJ (1969) Effects of carnosine and anserine on muscle adenosine triphosphatases. J Biol Chem 244:1600–1604
Bate-Smith EC (1938) The buffering of muscle in rigor: protein, phosphate and carnosine. J Physiol (Lond) 92:336–343
Bergstrom J (1962) Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest 14 [Suppl 68]:l-110
Bergstrom J, Fürst P, Noree L-O, Vinnars E (1978) Intracellular free amino acids in muscle tissue of patients with chronic uraemia: effect of peritoneal dialysis and infusion of essential amino acids. Clin Sci Mol Med 54:51–60
Boldyrev AA (1986) Biological significance of histidine-containing dipeptides. Biokhimiia 51:1930–1943
Boldyrev AA, Dupin AM, Pindel EV, Severin SE (1988) Antioxidative properties of histidine-containing dipeptides from skeletal muscles of vertebrates. Comp Biochem Physiol [B] 89:245–250
Christman AA (1976) Factors affecting anserine and carnosine levels in skeletal muscles of various animals. Int J Biochem 7:519–527
Crush KG (1970) Carnosine and related substances in animal tissues. Comp Biochem Physiol 34:3–30
Davey CL (1960) The significance of carnosine and anserine in striated skeletal muscle. Arch Biochem Biophys 89:303–308
Downie NM, Heath RW (1974) Basic statistical methods, 4th edn. Harper and Row, New York
Harris RC, Hultman E, Nordesjo L-O (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33:109–120
Harrison SM, Lamont C, Miller DJ (1985) Carnosine and other natural imidazoles enhance muscle calcium sensitivity and are mimicked by caffeine and AR-L 115BS. J Physiol (Lond) 371:197P
Johnson P, Fedyna JS, Schindzielorg A, Moritz-Smith C, Rasvinsky PJ (1982) Regulation of muscle phosphorylase activity by carnosine and anserine. Biochem Biophys Res Commun 109:769–775
Mannion AF, Jakeman PM, Willan PLT (1990) The measurement of skeletal muscle buffer value in man. J Physiol (Lond) 429:109P
Marlin DJ, Harris RC, Snow DH (1988) Muscle buffering capacity and carnosine contents in the thoroughbred horse. Can J Sport Sci 13:24P
Marlin DJ, Harris RC, Gash SP, Snow DH (1989) Carnosine content of the middle gluteal muscle in thoroughbred horses with relation to age, sex and training. Comp Biochem Physiol [A] 93:629–632
Parker CJ, Ring E (1970) A comparative study of the effect of carnosine on myofibrillar ATPase activity of vertebrate and invertebrate muscles. Comp Biochem Physiol 37:413–419
Parkhouse WS, McKenzie DC, Hochachka RW, Ovalle WK (1985) Buffering capacity of deproteinised human vastus lateralis muscle. J Appl Physiol 58 (1):14–17
Safrit M (1981) Evaluation in physical education, 2nd edn. Prentice Hall, Englewood Cliffs, NJ
Simoneau JA, Bouchard D (1989) Human variation in skeletal muscle fibre-type proportion and enzyme activities. Am J Physiol 257:E567-E572
Tanokura M, Tasumi M, Miyazawa T (1976) 1H nuclear magnetic resonance studies of histidine-containing di and tripeptides. Estimation of the effects of charged groups on the pKa' value of the imidazole ring. Biopolymers 15:393–401
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mannion, A.F., Jakeman, P.M., Dunnett, M. et al. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Europ. J. Appl. Physiol. 64, 47–50 (1992). https://doi.org/10.1007/BF00376439
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00376439