Summary
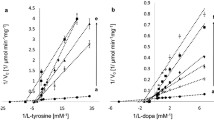
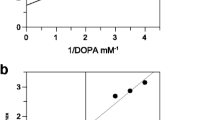
Azelaic acid, and other saturated dicarboxylic acids (C9-C12), are shown to be competitive inhibitors of tyrosinase (K I azelaic acid = 2.73×10−3 M) and of membrane-associated thioredoxin reductase (K I azelaic acid = 1.25×10−5 M). The monomethyl ester of azelaic acid does not inhibit thioredoxin reductase, but it does inhibit tyrosinase, although double the concentration is necessary compared with azelaic acid (K I azelaic acid monomethyl ester = 5.24×10−3 M). Neither azelaic acid nor its monomethyl ester inhibit tyrosinase when catechol is used as a substrate instead of l-tyrosine. Therefore, the weak inhibitory action of azelaic acid on tyrosinase appears to be due to the competition of a single carboxylate group on this inhibitor for the α-carboxylate binding site of the l-tyrosine substrate on the enzyme active site. Based on the inhibitor constant on tyrosinase, at least cytotoxic levels of azelaic acid would be required for the direct inhibition of melanin biosynthesis in melanosomes if this mechanism is responsible for depigmentation in the hyperpigmentation disorders lentigo maligna and melasma. Alternatively only 10−5 M azelaic acid is required to inhibit thioredoxin reductase. This enzyme is shown to regulate tyrosinase through a feedback mechanism involving electron transfer to intra-cellular thioredoxin, followed by a specific interaction between reduced thioredoxin and tyrosinase. Furthermore, the thioredoxin reductase/thioredoxin system is shown to be a principal electron donor for the ribonucleotide reductases which regulates DNA synthesis. Inhibition of thioredoxin reductase by azelaic acid provides a rationale for both its depigmenting property and the reversible inhibition of DNA synthesis observed in cultured epidermal cells and also in some of the bacteria associated with acne vulgaris.
Similar content being viewed by others
References
Breathnach AS, Ward BJ, Robins EJ, Bashin Y, Ethridge L, Passi S, Nazzaro-Porro M (1985) Analytic ultrastructural auto-radiographic localization by 3H dicarboxylic acids in cultured normal human melanocytes and keratinocytes. In: Bagnara J, Klaus SN, Paul E, Schartl M (eds) Biological, molecular, and clinical aspects of pigmentation. XIIth International Pigment Cell Conference. University of Tokyo Press, pp 627–641
Doherty VR, Ashworth J, Cox N (1987) Azelaic acid in lentigo maligna. Br J Dermatol 116:606
Duckworth HW, Coleman JE (1970) Physicochemical and kinetic properties of mushroom tyrosinase. Biol Chem 245:1613–1619
Fuchs J (1977) Isolation of an Escherichia coli mutant deficient in thioredoxin reductase. J Bacteriol 129:967–974
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237–271
Holmgren A, Branden C-I, Jornvall H, Sjoberg B-M (1986) Thioredoxin and glutaredoxin systems: structure and function. Raven Press, New York
Kwon BS, Hag AK, Pomerantz SH, Halaban R (1987) Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci USA 84:7474–7479
Leeming JP, Holland KT, Bojar RA (1986) The in vitro antimicrobial effect of azelaic acid. Br J Dermatol 115:551–558
Leibl H, Stingl G, Pehamburger H, Korchan H, Konrad K, Wolf K (1985) Inhibition of DNA synthesis of melanoma cells by azelaic acid. J Invest Dermatol 85:417–422
Lerch K (1988) Protein and active site structure of tyrosinase. In: Bagnara JT (ed) Advances in pigment cell research. Prog Clin Biol Res 256:88–97
Mason HS (1957) Mechanism of oxygen metabolism. Adv Enzymol 19:79–86
McLean DI, Peter KK (1986) Apparent progression of lentigo maligna to invasive melanoma during treatment with topical azelaic acid. Br J Dermatol 114:685–689
Nazzaro-Porro M, Passi S (1978) Identification of tyrosinase inhibitors in cultures of pityrosporum. J Invest Dermatol 71:205–208
Nazzaro-Porro M, Passi S (1979) Azelaic acid as a competitive inhibitor of tyrosinase. Pigment Cell 4:234–243
Nazzaro-Porro M, Passi S, Balus L, Breathnach AS (1979) Effect of dicarboxylic acids on lentigo maligna. J Invest Dermatol 72:296–305
Norris J, Cunliffe WJ, Burke B (1987) Azelaic acid really does work in acne — a double-blind national and international study. Br J Dermatol 117:34–35
Pathak MA, Wick M, Farinelli W, Fitzpatrick TB (1979) Evaluation of azelaic acid on normal skin pigmentation and on B16 mouse melanoma. J Invest Dermatol 72:266
Robins EJ, Breathnach AS, Bashin VP, Ethridge L, Nazzaro-Porro M, Passi S, Picardo M (1986) Azelaic acid. J Invest Dermatol 87:293–294
Schallreuter KU, Pittelkow MR (1988) Defective calcium uptake system in vitiligo. Arch Dermatol Res 280:137–139
Schallreuter KU, Witkop CJ Jr (1988) Thioredoxin reductase activity in Hermansky-Pudlak syndrome. A method for identification of putative heterozygotes. J Invest Dermatol 90:372–377
Schallreuter KU, Wood JM (1986) The role of thioredoxin reductase in the reduction of free radicals at the surface of the epidermis. Biochem Biophys Res Commun 136:630–637
Schallreuter KU, Wood JM (1987) Azelaic acid as a competitive inhibitor of thioredoxin reductase in human melanoma cells. Cancer Lett 36:297–305
Schallreuter KU, Wood JM (1988) The activity and purification of membrane-associated thioredoxin reductase from human metastatic melanotic melanoma. Biochim Biophys Acta 967:103–109
Schallreuter KU, Wood JM (1989) Free radical reduction in the human epidermis. Free Radic Biol Med 6:519–531
Schallreuter KU, Wood JM (1989) Thioredoxin reductase and its clinical significance in control of the pigmentary system in clinical aspects of pigmentary disorders. Clin Dermatol 7:92–105
Schallreuter KU, Wood JM (1989) Calcium regulates thioredoxin reductase in human metastatic melanoma. Biochim Biophys Acta 997:242–247
Schallreuter KU, Pittelkow MR, Gleason FK, Wood JM (1986) The role of calcium in the regulation of free radical reduction by thioredoxin reductase at the surface of the skin. Inorganic Biochem 28:227–238
Schallreuter KU, Schulz KH, Wood JM (1986) The induction of contact dermatitis in guinea pigs by quaternary ammonium compounds: the mechanism of antigen formation. Environ Health Perspect 70:229–237
Schallreuter KU, Pittelkow MR, Wood JM (1986) Free radical-reduction by thioredoxin reductase at the surface of normal and vitiliginous keratinocytes. J Invest Dermatol 87:728–732
Schallreuter KU, Hordinsky MR, Wood JM (1987) Thioredoxin reductase: free radical reduction by thioredoxin reductase on the skin of different hypopigmentation disorders. Arch Dermatol 123:615–619
Schallreuter KU, Wood JM, Breitbart EW, Kimmig W, Hicks R, Radloff H, JÄnner M (1987) Thioredoxin reductase in primary melanoma and surrounding skin. European Society for Pigment Cell Research, Sorrento, Italy, Proceedings, pp 41, 95
Wood JM, Schallreuter KU (1988) Reduced thioredoxin inhibits melanin biosynthesis: evidence for the formation of a stable bis-cysteinate complex with tyrosinase. Inorganica Chem Acta 151:7
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schallreuter, K.U., Wood, J.W. A possible mechanism of action for azelaic acid in the human epidermis. Arch Dermatol Res 282, 168–171 (1990). https://doi.org/10.1007/BF00372617
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00372617