Abstract
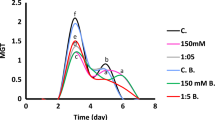
In order to study the establishment of a spermosphere, the C2H2 reduction activity of N2-fixing bacteria isolated from river sand was examined in a simulated spermosphere in the river sand which contained sucrose, an amino acid mixture, and CN- released from plant seeds. The sand incubated with 10-10 to 10-9 mol CN- 30 g sand-1 exhibited higher C2H2 reduction activity than that without CN-. The change in the most probable number of N2 fixers with increasing quantities of CN- roughly corresponded to that in C2H2 reduction activity. However, the most probable number of non-N2-fixing bacteria decreased except for CN--tolerant ones. Both C2H2 reduction activity and proliferation of the N2 fixers isolated on a modified Burk's medium were almost similar to those in the bacteria in the sand. In contrast, the proliferation of some nonfixers decreased with an increasing CN- concentration. C2H2 reduction activity of N2 fixers cultured in combination with non-fixers exhibited a clear peak at 10-7 M CN- as for C2H2 reduction activity in the sand. We therefore speculate that cyanide evolved from seeds during a pregermination period may suppress the growth of general bacteria, but may promote the proliferation of N2 fixers, thus contributing to the establishment of a spermosphere.
Similar content being viewed by others
References
Abbot TP, Nakamura LK, Nelsen TC, Gasdorf HJ, Bennett GA, Kleiman R (1990) Microorganisms for degrading simmondsin and related cyanogenic toxins in jojoba. Appl Microbiol Biotechnol 34:270–273
van Berkum P, Sloger C (1979) Immediate acetylene reduction by excised grass root not previously preincubated at low oxygen tensions. Plant Physiol 64:739–743
Conn EE (1980) Cyanogenic compounds. Annu Rev Plant Physiol 31:433–451
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Esashi Y, Isuzugawa K, Matsuyama S, Ashino H, Hasegawa R (1991) Endogenous evolution of HCN during pre-germination periods in many seed species. Physiol Plant 83:27–33
Harris R, Knowles CJ (1983) Isolation and growth of a Pseudomonas species that utilizes cyanide as a source of nitrogen. J Gen Microbiol 129:1005–1011
Hwang JC, Burris RH (1972) Nitrogenase-catalyzed reaction. Biochim Biophys Acta 283:339–350
Li J-G, Burgess BK, Corbin JL (1982) Nitrogenase-reactivity: Cyanide as substrate and inhibitor. Biochem 21:4393–4402
Lowe DJ, Fisher K, Thorneley RNF, Vaughn SA, Burgess BK (1989) Kinetics and mechanism of the reaction of cyanide with molybdenum nitrogenase from Azotobacter vinelandii. Biochem 28:8460–8466
Nawaz MS, Davis JW, Wolfram JH, Chapatwala KD (1991) Degradation of organic cyanides by Pseudomonas aeruginosa. Appl Biochem Biotechnol 28/29:865–875
Ota H, Kurihara Y, Satoh S, Esashi Y (1991) Development of acetylene reduction (nitrogen-fixation) activity on and around imbibed plant seeds. Soil Biol Biochem 23:9–14
Rivera-Ortiz JM, Burris RH (1975) Interaction among substrates and inhibitors of nitrogenase. J Bacteriol 123:537–545
Silva-Avalos J, Richmond MG, Nagappan O, Kunz DA (1990) Degradation of the metal-cyano complex tetracyanonickelate(II) by cyanide-utilizing bacterial isolates. Appl Environ Microbiol 56:3664–3670
Spackman DH, Stein WH, Moore S (1958) Automatic recording apparatus for use in the chromatography of amino acid. Anal Chem 30:1190–1206
Taylor J (1962) The estimation of numbers of bacteria by tenfold dilution series. J Appl Bact 25:54–61
Wilson PW, Knight SG (1952) Experiments in bacterial physiology, 3rd. edn. Burgess, Minneapolis, Minn
Yemm EW, Cocking EC (1955) The determination of amino acids with ninhydrin. Analyst 80:209–213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ota, H., Easashi, Y. Use of NaCN to increase the growth of N2-fixing bacteria in a model spermosphere mimicked by sucrose and amino acids leached by seeds. Biol Fertil Soils 22, 305–309 (1996). https://doi.org/10.1007/BF00334574
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00334574