Abstract
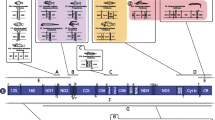
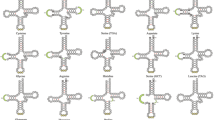
We amplified and sequenced the mitochondrial control region from 23 species representing six families of teleost fish. The length of this segment is highly variable among even closely related species due to the presence of tandemly repeated sequences and large insertions. The position of the repetitive sequences suggests that they arise during replication both near the origin of replication and at the site of termination of the D-loop strand. Many of the conserved sequence blocks (CSBs) observed in mammals are also found among fish. In particular, the mammalian CSB-D is present in all of the fish species studied. Study of potential secondary structures of RNAs from the conserved regions provides little insight into the functional constraints on these regions. The variable structure of these control regions suggests that particular care should be taken to identify the most appropriate segment for studies of intraspecific variation.
Similar content being viewed by others
References
Avise JC, Ball RM, Arnold J (1988) Current versus historical population sizes in vertebrate species with high gene flow: a comparison based on mitochondria DNA lineages and inbreeding theory for neutral mutations. Mol Biol Evol 5:331–344
Bartlett SE, Davidson WS (1991) Identification of Thunnus tuna species by the polymerase chain reaction and direct sequence analysis of their mitochondrial cytochrome b genes. Can J Fish Aquat Sci 48:309–317
Beckenbach AT (1991) Rapid mtDNA sequence analysis of fish populations using the polymerase chain reaction (PCR). Can J Fish Aquat Sci 48(Suppl 1):95–98
Bibb MJ, Etten RA, Wright CT, Walberg MW, Clayton DA (1981) Sequence and gene organization of mouse mitochondrial DNA. Cell 26:167–180
Birky CW Jr., Fuerst P, Maruyama T (1989) Organelle gene diversity under migration, mutation, and drift: equilibrium expectations, approach to equilibrium, effects of heteroplasmic cells, and comparison to nuclear genes. Genetics 121:613–627
Billington N, Hebert PDN (1991) Mitochondrial DNA diversity in fishes and its implications for introductions. Can J Fish Aquat Sci 48:80–94
Brown WM (1985) The mitochondrial genome of animals. In: MacIntyre RJ (ed) Molecular evolutionary genetics. Plenum Press, New York, pp 95–130
Brown WM, George M JR, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Pro Nat Acad Sci U S A 76:1967–1971
Buroker NE, Brown JR, Gilbert TA, O'Hara PJ, Beckenbach AT, Thomas WK, Smith MJ (1990) Length heteroplasmy of sturgeon mitochondrial DNA: an illegitimate elongation model. Genetics 124:157–63
Cabot EL, Beckenbach AT (1989) Simultaneous editing of multiple nucleic acid and protein sequences with ESEE. Comput Appl Biosci 5:233–234
Carr SM, Marshall HD (1991a) Detection of DNA sequence variation in the mitochondrial cytochrome b gene of Atlantic cod (Gadus morhua) by the polymerase chain reaction. Can J Fish Aquat Sci 48:48–52
Carr SM, Marshall HD (1991b) A direct approach to the measurement of genetic variation in fish populations: applications of the polymerase chain reaction to studies of Atlantic cod, Gadus morhua L. J Fish Bio 39(A):101–107
Carrel RL (1988) Vertebrate paleontology and evolution. W.H. Freeman. New York
Chang DD, Clayton DA (1986) Identification of primary transcription start sites of mouse mitochondrial DNA: accurate in vitro initiation of both heavy- and light-strand transcriptions. Mol Cell Biol 6: 1446–1453
Chang Y-S, Huang F-I, Lo T-B (1994) The complete nucleotide sequence and gene organization of carp (Cyprinus carpio) mitochondrial genome. J Mol Evol 38:138–155
Clayton DA (1982) Replication of animal mitochondrial DNA. Cell 28:693–705
Doda IN, Wright CT, Clayton DA (1981) Elongation of displacementloop strands in human and mouse mitochondrial DNA is arrested near specific template sequences. Proc Natl Acad Sci U S A 78(10): 6116–6120
Finnerty JR, Block BA (1992) Direct sequencing of mitochondrial DNA detects highly divergent haplotypes in blue marlin (Makaira nigricans). Mol Mar Biol Biotech 1:206–214
Gauldie RW (1991) Taking stock of genetic concepts in fisheries management. Can J Fish Aquat Sci 48:722–731
Graves JE, McDowell JR, Beardsley AM, Scoles DR (1992) Stock structure of the bluefish Pomatostomus saltatrix along the mid-Atlantic coast. Fish Bull 90:703–710
Hoelzel AR, Hancock JM, Dover GA (1991) Evolution of the cetacean mitochondrial D-Loop region. Mol Biol Evol 8(3):475–493
Johansen S, Guddal PH, Johansen T (1990) Organization of the mitochondrial genome of Atlantic cod, Gadus morhua. Nucleic Acids Res 18:411–419
Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, Villablanca FX, Wilson AC (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A 86:6196–6200
Kocher TD, Wilson AC (1991) Sequence evolution of mitochondrial DNA in human and chimpanzees. In: Osawa S, Honjo T (eds) Evolution of life: fossils, molecules and culture. Springer-Verlag, Tokyo, pp 391–413
Kumar S, Tamura K, Nei M (1993) MEGA: molecular evolutionary genetics analysis, version 1.0. The Pennsylvania State University, University Park, PA 16802
Lauder GV, Liem KF (1983) The evolution and interrelationships of the actinopterygian fishes. Bull Mus Comp Zool 150(3):95–197
Mignotte B, Dunen-Bluteau D, Reiss C, Mounolou J-C (1987) Sequence deduced physical properties in the D-loop region common to five vertebrate mitochondrial DNAs. J Theor Biol 124:57–69
Ovenden JR (1990) Mitochondrial DNA and marine stock assessment: A review. Aust J Mar Freshwater Res 41:835–853
Saccone C, Attimonelli M, Sbisá E (1987) Structural elements highly preserved during the evolution of the D-loop-containing region in vertebrate mitochondrial DNA. J Mol Evol 26:205–211
Saccone C, Pesole G, Sbisá E (1991) The main regulatory region of mammalian mitochondrial DNA: Structure-function model and evolutionary pattern. J Mol Evol 33:83–91
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, NY
Saitou N, Nei M (1987) The neighbor joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–1125
Shedlock AM, Parker JD, Crispin DA, Pietsch TW, Burner GC (1992) Evolution of the salmonid mitochondrial control region. Mol Phyl Evol 1:179–192
Shields GF, Kocher TD (1991) Phylogenetie relationships of North American ursids based on analysis of mitochondrial DNA. Evolution 45:218–221
Southern SO, Southern PJ, Dizon AE (1988) Molecular characterization of a cloned dolphin mitochondrial genome. J Mol Evol 28:32–42
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tzeng C-S, Hui C-F, Shen S-C, Huang PC (1992) The complete nucleotide sequence of the Crossostoma lacustre mitochondrial genome: conservation and variations among vertebrates. Nucleic Acids Res 20(18):4853–4858
Wilson AC, Cann RL, Carr SM, George M, Gyllensten UB, Helm-Bychowski KM, Higuchi RG, Palumbi SR, Prager EM, Sage RD, Stoneking M (1985) Mitochondrial DNA and two perspectives on evolutionary genetics. Biol J Linn Soci 26:375–400
Wong JF, Ma EP, Wilson RK, Roe BA (1983) DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res 11(14):4977–4995
Author information
Authors and Affiliations
Additional information
Correspondence to: T.D. Kocher
Rights and permissions
About this article
Cite this article
Lee, WJ., Conroy, J., Howell, W.H. et al. Structure and evolution of teleost mitochondrial control regions. J Mol Evol 41, 54–66 (1995). https://doi.org/10.1007/BF00174041
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174041