Summary
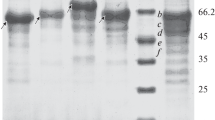
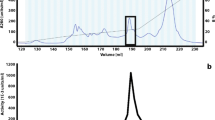
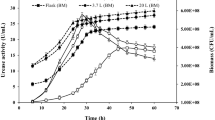
Acid urease was purified to an electrophoretically homogeneous state, and the molecular weight was estimated to be 220 000. The enzyme consisted of three kinds of subunits, designated α, β and λ, with molecular weights of 67 000, 16 800 and 8600, respectively, in a (α1 \2λ1)2 structure. The isoelectric point of the enzyme was 4.8. The nickel content was found to be 1.9 atoms of nickel per α1β2λ1 unit. The amino acid profile was different from those of known bacterial neutral ureases. The enzyme was most active at pH 2 and around 65° C. It was stable between pH 3 and 9, and below 50° C. The K m for urea was 2.7 mM at pH 2. The enzyme activity was inhibited by Ag+, Hg2+, Cu2+, p-chloromercuribenzoate and acetohydroxamate. The enzyme was separated into three subunits by reverse phase HPLC. The amino terminal amino acid sequences of the subunits α, β and λ were Ser-Phe-Asp-Met-, Met-Val-Pro-Gly- and Met-Arg-Leu-Thr-, respectively.
Similar content being viewed by others
References
Bast E (1988) Nickel requirement for the formation of active urease in purple sulfur bacteria. Arch Microbiol 150:6–10
Christians S, Kaltwasser H (1986) Nickel-content of urease from Bacillus pasteurii. Arch Microbiol 145:51–55
Dixon M, Webb EC (1979) “Enzymes,” 3rd edn. Longman Group, London
Dixon NE, Gazzola C, Blakeley RL, Zerner B (1975) Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 97:4131–4133
Dixon NE, Hinds JA, Fihelly AK, Gazzola C, Winzor DJ, Blakeley RL, Zerner B (1980) Jack bean ureases. IV. The molecular size and the mechanism of inhibition by hydroxamic acid. Can J Biochem 58:1323–1334
Hausinger RP (1986) Purification of a nickel-containing urease from the rumen anaerobe Selenomonas ruminantium. J Biol Chem 261:7866–7870
Jones BD, Mobley HLT (1988) Proteus mirabilis urease: genetic organization, regulation and expression of structural genes. J Bacteriol 170:3342–3349
Kakimoto S, Okazaki K, Sakane Y, Imai K, Sumino Y, Akiyama S, Nakao Y (1989a) Isolation and taxonomic characterization of acid urease-producing bacteria. Agric Biol Chem 53:1111–1117
Kakimoto S, Sumino Y, Akiyama S, Nakao Y (1989b) Purification and characterization of acid urease from Lactobacillus reuteri. Agric Biol Chem 53:1119–1125
Mackerras AH, Smith GD (1986) Urease activity of the cyanobacterium Anabaena cylindrica. J Gen Microbiol 132:2749–2752
Mamiya G, Takishima K, Masakuni M, Kayumi T, Ogawa K, Sekita T (1985) Complete amino acid sequence of jack bean urease. Proc Jpn Acad 61:395–398
Mobley HLT, Hausinger RP (1989) Microbial ureases: significance, regulation and molecular characterization. Microbiol Rev 53:85–108
Mobley HLT, Jones BD, Jerse AE (1986) Cloning of urease gene sequences from Providencia stuartii. Infect Immun 54:161–169
Moreau MC, Ducluzeau R, Raibaud P (1976) Hydrolysis of urea in the gastrointestinal tract of “monoxenic” rats: effect of immunization with strains of ureolytic bacteria. Infect Immun 13:9–15
Mulrooney SB, Lynch MJ, Mobley HLT, Hausinger RP (1988) Purification, characterization and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol 170:2202–2207
Nakano H, Takenishi S, Watanabe Y (1984) Purification and properties of urease from Brevibacterium ammoniagenes. Agric Biol Chem 48:1495–1502
Ough CS (1976) Ethylcarbamate in fermented beverages and foods. J Agric Food Chem 24:323–328
Polacco JC, Havir EA (1979) Comparison of soy bean urease isolated from seed and tissue culture. J Biol Chem 254:1707–1715
Schneider J, Kaltwasser H (1984) Urease from Arthrobacter oxydance, a nickel-containing enzyme. Arch Microbiol 139:355–360
Suzuki K, Benno Y, Mitsuoka T, Takebe S, Kobashi K, Hase J (1979) Urease-producing species of intestinal anaerobes and their activities. Appl Environ Microbiol 37:379–382
Takebe S, Kobashi K (1988) Acid urease from Lactobacillus of rat intestine. Chem Pharm Bull 36:693–699
Todd MJ, Hausinger RP (1987) Purification and characterization of nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem 262:5963–5967
Yoshizawa K, Takahashi K (1988) Utilization of urease for decomposition of urea in sake. J Brew Soc Jpn 83:142–144
Author information
Authors and Affiliations
Additional information
Offprint requests to: S. Kakimoto
Rights and permissions
About this article
Cite this article
Kakimoto, S., Sumino, Y., Kawahara, K. et al. Purification and characterization of acid urease from Lactobacillus fermentum . Appl Microbiol Biotechnol 32, 538–543 (1990). https://doi.org/10.1007/BF00173724
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173724