Summary
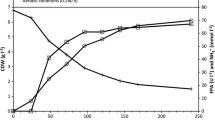
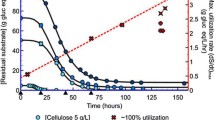
Clostridium thermocellum produced different levels of true cellulase (“Avicelase”) depending on the carbon source used for growth. In defined medium with fructose, the cellulase titer was seven times higher than with cells growing on cellobiose and four times higher than cells growing with glucose. During the lag phase on fructose, the differences were even more dramatic, i.e. 60 times higher than in cells growing on cellobiose and 40 times that of cells lagging or growing in glucose. In an attempt to detect factors that might contribute to these differences, we considered intracellular ATP, chemical potential (ΔpH), electrical potential (ΔY), proton motive force (Δp), growth rate, and rates of uptake of inorganic phosphate and sugars. We noted a direct correlation between cellulase production and intracellular ATP levels and an inverse relationship of cellulase production with ΔY and Δp values. It thus appears that cellulase is best produced by cells high in ATP and low in Dp and its electrical component DY. There was no obvious relationship between the cellulase titer and the other parameters. Although the physiological significance of such correlations is unknown, the data suggest that further investigation is warranted.
Similar content being viewed by others
References
Alexander, J.K. 1968. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J. Biol. Chem.243: 2899–2904.
Beguin, P., J. Millet, and J.-P. Aubert. 1992. Cellulose degradation by Clostridium thermocellum: From manure to molecular biology. FEMS Microbiol. Lett.100: 523–528.
Blaut, M., and G. Gottschalk. 1984. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur. J. Biochem.141: 217–222.
Blaut, M., V. Muller, K. Fiebig, and G. Gottschalk. 1985. Sodium ions and an energized membrane required by Methanosarcina barkeri for the oxidation of methanol to the level of formaldehyde. J. Bacteriol.164: 95–101.
Buchanan, R. L., S. B. Jones, W. V. Gerasimowicz, L. L. Zaika, H. G. Stahl, and L. A. Ocker. 1987. Regulation of aflatoxin biosynthesis: assessment of the role of cellular energy status as a regulation of the induction of aflatoxin production. Appl. Environ. Microbiol.53: 1224–1231.
Grepinet, O., M.-C. Chebrou, and P. Beguin. 1988. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J. Bacteriol.170: 4582–4588
Johnson, E. A. 1983. Regulation of cellulase activity and synthesis in Clostridium hermocellum. Ph. D. thesis, M. I. T., Cambridge, MA.
Johnson, E. A., A. Madia, and A. L. Demain. 1981. Chemically defined minimal medium for growth of the anaerobic cellulolytic thermophile Clostridium thermocellum. Appl. Environ. Microbiol.41:1060–1062.
Johnson, E. A., M. Sakajoh, G. Halliwell, A. Madia, and A. L. Demain. 1982. Saccharification of complex cellulosic substrates by the cellulase system from Clostridium thermocellum. Appl. Environ. Microbiol.43: 1125–1132.
Johnson, E. A., Bouchot, F. A., and A. L. Demain. 1985. Regulation of cellulase formation in Clostridium thermocellum. J. Gen. Microbiol.131: 2303–2308.
Kadam, S., A. L. Demain, J. Millet, P. Beguin, and J.-P. Aubert. 1988. Molecular cloning of a gene for a thermostable β-glucosidase from Clostridium thermocellum into Escherichia coli. Enzyme Microb. Technol.10: 9–13.
Kashket, E. R., A. G. Blanchard, and W. C. Metzger. 1980. Proton motive force during growth of Streptococcus lactis cells. J. Bacteriol.143: 128–134.
Khan, S., and R. M. Macnab. 1980. Proton chemical potential, proton electrical potential and bacterial motility. J. Mol. Biol.138: 599–614.
Lamed, R., and J. G. Zeikus. 1980. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J. Bacteriol.156: 828–836.
Lamed, R., E. Setter, R. Kenig, and E. A. Bayer. 1983. The cellulosome -A discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities. Biotechnol. Bioeng. Symp.13: 163–181.
Mackenzie, C. R., R. C. A. Yang, G. B. Patel, D. Bilous, and S. A. Narang. 1989. Identification of three distinct Clostridium thermocellum xylanase genes by molecular cloning. Arch. Microbiol.152: 377–381.
Mayer, F., M. P. Coughlan, Y. Mori, and L. G. Ljungdahl. 1987. Macromolecular organization of the cellulolytic enzyme complex of Clostridium thermocellum as revealed by electron microscopy. Appl Environ. Microbiol.53: 2785–2792.
Muller, V., M. Blaut, and G. Gottschalk. 1987. Oxidation of trimethylamine to the level of formaldehyde by Methanosarcina barkeri is dependent on the protonmotive force. FEMS Microbiol Lett.43: 183–186.
Nochur, S. V., M. F. Roberts, and A. L. Demain. 1990. Mutation of Clostridium thermocellum in the presence of certain carbon sources. FEMS Microbiol. Lett.71: 199–204.
Nochur, S. V., A. L. Demain, and M. F. Roberts. 1992a. Carbohydrate utilization by Clostridium hermocellum: Importance of internal pH in regulating growth. Enzyme Microb. Technol.14: 338–349.
Nochur, S. V., G. R. Jacobson, M. F. Roberts, and A. L. Demain. 1992b. Mode of sugar phosphorylation in Clostridium thermocellum. Appl. Biochem. Biotechnol.33: 33–41.
Romaniec, M. P. M., K. Davidson, and G. P. Hazlewood. 1987. Cloning and expression in Escherichia coli of Clostridium thermocellum DNA encoding β-glucosidase activity. Enzyme Microb. Technol.9: 474–478.
Schimming, S., W. H. Schwarz and W. J. Staudenbauer. 1992. Structure of the Clostridium thermocellum gene licB and the encoded b-1,3–1,4 glucanase; a catalytic region homologous to Bacillus lichenase joined to the reiterated domain of clostridial cellulases. Eur. J. Biochem.204: 13–19.
Sheth, K., and J. K. Alexander. 1969. Purification and properties of beta-1,4-oligoglucan: orthophosphate glucosyltransferase from Clostridium thermocellum. J. Biol. Chem.244: 457–464.
Szmelcman, S., and J. Adler. 1976. Change in membrane potential during bacterial chemotaxis. Proc. Natl. Acad. Sci. USA.73: 4387–4391.
Zverlov, V. V., D. A. Laptev, V. I. Tishkov, and G. A. Velikodvorskaya. 1991. Nucleotide sequence of the Clostridium thermocellum laminarinase gene. Biochem. Biophys. Res. Commun.181: 507–512.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nochur, S.V., Roberts, M.F. & Demain, A.L. True cellulase production by Clostridium thermocellum grown on different carbon sources. Biotechnol Lett 15, 641–646 (1993). https://doi.org/10.1007/BF00138556
Issue Date:
DOI: https://doi.org/10.1007/BF00138556