Summary
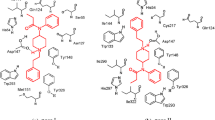
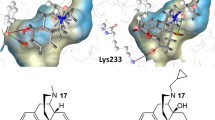
Molecular modeling studies were carried out on a set of piperazine and 3,8-diazabicyclo[3.2.1]octane derivatives with the aim to highlight the main factors modulating their affinity for the μ-opioid receptor. Structure-affinity relationships were developed with the aid of molecular mechanics and semiempirical quantum-mechanics methods. According to our proposed pharmacodynamic model, the binding to the μ-receptor is promoted by the following physico-chemical features: the presence of hydrocarbon fragments on the nitrogen ring frame capable of interacting with one of two hypothesized hydrophobic receptor pockets; a ‘correct’ orientation of an N-propionyl side chain so as to avoid a sterically hindered region of the receptor; the possibility of accepting a hydrogen bond from a receptor site complementary to the morphine phenol oxygen.
Similar content being viewed by others
References
Cignarella, G., Occelli, E., Cristiani, G.F., Paduano, L. and Testa, E., J. Med. Chem., 6 (1963) 764.
Cignarella, G. and Testa, E., J. Med. Chem., 11 (1968) 592.
Cignarella, G., Barlocco, D., Tranquillini, M.E., Volterra, A., Brunello, N. and Ragagni, G., Pharmacol. Res. Commun., 120 (1988) 383.
SYBYL Molecular Modeling System (version 5.41), TRIPOS Associates, 1699 South Hanley Road, Suite 303, St. Louis, MO 63144, U.S.A.
Vinter, J.G., Davis, A. and Saunders, M.J., J. Comput.-Aided Mol. Design, 1 (1987) 31.
MOPAC (version 5.00), Quantum Chemistry Program Exchange, No. 455, 1989.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
Toma, L., Cignarella, G., Barlocco, D. and Ronchetti, F., Tetrahedron, 48 (1992) 159.
Tai, J. and Allinger, N.L., J. Am. Chem. Soc. 110 (1988) 2050.
Marshall, G.R., Barry, C.D., Bosshard, R.A., Dammkoehler, R.A. and Dunn, D.A., In: Olson, E.C. and Christoffersen, R.E. (Eds.) Computer-Assisted Drug Design, (ACS Symposium No. 112), Washington, DC, 1979, pp. 205–226.
Clark, M.J., Carter, B.D. and Medzihradsky, F., Eur. J. Pharmacol. 148 (1988) 343.
Beckett, A.H. and Casy, A.F., J. Pharm. Pharmacol., 6 (1954) 986.
Feinberg, A.P., Creese, I. and Snyder, S.H., Proc. Natl. Acad. Sci. USA, 73 (1976) 4215.
Martin, W.R., Pharmacol. Rev., 35 (1983) 283.
Kolb, V.M., Adv. Drug. Res., 16 (1987) 281.
Bentley, K. and Lewis, J.W., In: Kosterlitz, H.W., Clouet, D.H. and Villarreal, J.E. (Eds.) Agonist and Antagonis Actions of Narcotic Analgesic Drugs, University Park Press, Baltimore, MD, 1973, pp. 7–16.
Smith, G.D. and Griffin, J.F., Science, 199 (1978) 1214.
Rice, K.C., Jacobson, A.E., BurkeJr., T.R., Bajwa, B.S., Streaty, R.A. and Klee, W.A., Science, 220 (1983) 314.
Lewis, J.W., Bentley, K.W. and Cowen, A., Annu. Rev. Pharmacol., 11 (1971) 241.
Kotick, M.P., Leland, D.L., Polazzi, J.O., Howes, J.F. and Bousquet, A., J. Med. Chem., 26 (1983) 1050.
Cometta-Morini, C., Maguire, P.A. and Loew, G.H., Mol. Pharmacol., 41 (1992) 185.
Cometta-Morini, C. and Loew, G.H., J. Comput.-Aided Mol. Design, 5 (1991) 335.
Sufrin, J., Dunn, D. and Marshall, G.R., Mol. Pharmacol., 19 (1981) 307.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barlocco, D., Cignarella, G., Greco, G. et al. Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo[3.2.1]octane derivatives binding to the μ-opioid receptor. J Computer-Aided Mol Des 7, 557–571 (1993). https://doi.org/10.1007/BF00124362
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00124362