Abstract
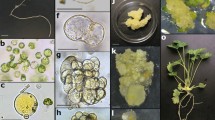
Plant regeneration from isolated protoplasts of 8 cultivars of lisianthus, Eustoma grandiflorum (Griseb.) Schinners, has been established by using activated charcoal. Protoplasts were isolated from lisianthus leaves grown in vitro and started to divide within 3–4 days of culture, but successful colony formation was only achieved by adding gellan gum blocks containing 1% (w/v) activated charcoal immediately after culture. Colonies consisting of as many as 50–100 cells formed after 30 days of culture and were transferred to fresh medium for callus proliferation and shoot regeneration, respectively. These shoots rooted on MS medium containing 0.5 mg l−1 indolebutyric acid(IBA) and the plantlets were finally transplanted to pots. Morphological characteristics, growth habit and pollen fertility of protoplast-derived plants of one cultivar were not different from those of seed-grown plants as control.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzylaminopurine
- NAA:
-
1-naphthaleneacetic acid
- MS:
-
Murashige & Skoog (1962) medium
- IBA:
-
indolebutyric acid
- MES:
-
2-N-morpholinoethane sulfonic acid
References
Binding H (1974) Regeneration of haploid and diploid from axenic culture of haploid Petnia hybrida L. Plant Sci. Lett. 2: 185–188
Carlberg I, Glimelius K & Eriksson T (1983) Improved culture ability of potato protoplasts by use of activated charcoal. Plant Cell Rep. 2: 223–225
Doughty S & Power JB (1988) Callus formation from leaf mesophyll protoplasts of Malus x domestica Borkh. cv. Greensleeves. Plant Cell Rep. 7: 200–201
Eriksson TR (1985) Protoplast isolation and culture. In: Fowke LC & Constabel F (eds) Plant Protoplast (pp 1–20) CRC Press, Boca Raton
Fridborg G & Eriksson T (1975) Effect of activated charcoal on growth and morphogenesis in cell cultures. Physiol. Plant. 34: 306–308
Fridborg G, Oedersen M, Landstrom LE & Eriksson T (1978) The effect of activated charcoal on tissue cultures; Adsorption of metabolites inhibiting morphogenesis. Physiol. Plant, 43: 104–106
Griesbach RJ & Semeniuk P (1987) Use of somaclonal variation in the improvement of Eustoma grandiflorum. J. Heredity 78: 114–116
Halevy AH & Kofranek AM (1984) Evaluation of Lisianthus as a new flower crop. HortScience 19: 845–847
Hu CY & Wang PJ (1983) Basic technique of plant cell culture, 5: Meristem, shoot tip, and bud culture, In: Evans DA, Sharp WA, Ammirato PV & Yamada Y (eds) Handbook of Plant Cell Culture, Vol 1 (pp 177–227). Macmillan Publ Co, New York
Kao KN & Michayluk MR (1974) A methods for high-frequency intergeneric fusion of plant protoplasts. Planta 115: 355–367
Kouider M, Hauptmann R, Widholm JM, Skirvin RM & Korban SS (1984) Callus formation from Malus x domestica cv. Jonathan protoplasts. Plant Cell Rep. 3: 142–145
Kozai T & Iwanami Y (1988) Effect of CO2 enrichment and sucrose concentration under high photon fluxes on plantlet growth of carnation (Dianthus caryophyllus L.) in tissue culture during the preparation stage. J Japan Soc. Hort. Sci. 57(2): 279–288
Kunitake H & Mii M (1990a) Somatic embryogenesis and plant regeneration from protoplasts of asparagus (Asparagus officinalis L.). Plant Cell Rep. 8: 706–710
Kunitake H & Mii M (1990b) Plant regeneration from cell culture-derived protoplasts of statice (Limonium perezii Hubbard). Plant Sci. 70: 115–119
Murashige T & Skoog F (1962) A revised medium for rapid growth and boiassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Niizeki M, Hidano Y & Saito K (1983) Callus formation from isolated protoplasts of apple, Malus pumila Mill. Japan J. Breed. 33: 369–374
O'Brien IEW & Lindsay GC (1993) Protoplasts to plants of Gentianaceae, Regeneration of lisianthus (Eustoma grandiflorum) is affected by calcium ion preconditioning, osmolality and pH of the culture media. Plant Cell, Tiss. Org. Cult. 33: 31–37.
Ochatt SJ & Caso OH (1986) Shoot regeneration from leaf mesophyll protoplasts of wild pear (Pyrus communis var. pyraster L.). J. Plant Physiol. 122: 243–249
Ochatt SJ & Power JB (1988) An alternative approach to plant regeneration from protoplasts of sour cherry (Prunus cerasus L.) Plant Sci. 56: 75–79
Oka S & Ohyama K (1985) Plant regeneration from leaf mesophyll protoplasts of Broussonetia kazinoki Sieb. (paper mulberry). J Plant Physiol. 119: 455–460
Semeniuk P & Griesbach RJ (1987) In vitro propagation of prairie gentian. Plant Cell Tiss. Org. Cult. 8: 249–253
Shepard JF & Totten RE (1977) Mesophyll cell protoplasts of potato. isolation, proliferation, and plant regeneration. Plant Physiol. 60: 313–316
Takahata Y & Jomori H (1989) Plant regeneration from mesophyll protoplasts of gentiana (Gentiana scabra Bungei). Plant Tissue Cult. Lett. 6(1): 19–21
Uematsu T (1989) prairie gentian. In: Matsuo T (ed) Collected Data of Plant Genetic Resources (pp 993–994), Kohdansha Scientific, Japan
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Tech. 47(4): 189–194
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kunitake, H., Nakashima, T., Mori, K. et al. Plant regeneration from mesophyll protoplasts of lisianthus (Eustoma grandiflorum) by adding activated charcoal into protoplast culture medium. Plant Cell Tiss Organ Cult 43, 59–65 (1995). https://doi.org/10.1007/BF00042672
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042672