Summary
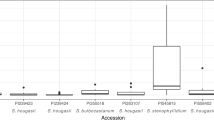
Over 5000 plants from 64 tuber-bearing wild Solanum spp. have been individually screened for resistance to Meloidogyne chitwoodi, M. fallax and M. hapla. Seedlings were analyzed by means of counting number of egg masses and resistance was verified by retesting low-scoring plants using stem cuttings. Resistance to both M. chitwoodi and M. fallax was observed in S. bulbocastanum, S. cardiophyllum, S. brachistotrichum, S. fendleri and S. hougasii. Only in S. chacoense and to a lesser extent in S. stoloniferum and S. gourlayi differential results between M. chitwoodi and M. fallax were observed. Resistance to M. hapla was found in S. bulbocastanum, S. brachistotrichum, S. cardiophyllum, S. arnezii, S. chacoense, S. tarijense, S. boliviense, S. gourlayi, S. microdontum, S. sparsipilum, S. spegazzinii, S. sucrense, S. acaule and S. hougasii. The occurrence of resistance in wild Solanum species in relation to their taxonomic status and the implications for introgression of resistance into S. tuberosum are discussed.
Similar content being viewed by others
References
Brown, C.R., H., Mojtahedi & G.S., Santo, 1989. Comparison of reproductive efficiency of Meloidogyne chitwoodi on Solanum bulbocastanum in soil and in vitro tests. Plant Disease 73: 957–959.
Brown, C.R., H., Mojtahedi & G.S., Santo, 1991. Resistance to Columbia root-knot nematode in Solanum spp. and in hybrids of S. hougasii with tetraploid cultivated potato. American Potato Journal 68: 445–452.
Brown, C.R., H., Mojtahedi, G.S., Santo & S., Austin-Phillips, 1994. Enhancing resistance to root-knot nematodes derived from wild Solanum species in potato germplasm. In: G.W., Zehnder, M.L., Powelson, R.K., Jansson & K.V., Raman (Eds.), Advances in Potato Pest Biology and Management, pp. 426–438. Raman. APS press, St. Paul.
Brücher, H., 1967. Rootknot-eelworm resistance in some South American tuber-forming Solanum species. American Potato Journal 44: 370–375.
Cuevas, J., O.Y. & C., Sosa-Moss, 1990. Host plants of Meloidogyne chitwoodi in the states of Tlaxcala and Puebla, Mexico. Current Nematology 1: 69–70.
Daykin, M.E. & R.S., Hussey, 1985. Staining and histopathological techniques in nematology. In: J.N., Sasser & C.C., Carter (Eds.), An Advanced Treatise on Meloidogyne. Vol. 2. Methodology, pp. 39–48. North Carolina State University Graphics, Raleigh.
Esbenshade, P.R. & A.C., Triantaphyllou, 1990. Isozyme phenotypes for the identification of Meloidogyne species. Journal of Nematology 22: 10–15.
Golden, A.M., J.H., O'Bannon, G.S., Santo & A.M., Finley, 1980. Description and SEM observations of Meloidogyne chitwoodi n.sp. (Meloidogynidae), root-knot nematode on potato in the Pacific Northwest. Journal of Nematology 12: 319–327.
Griffin, G.D., 1988. The Colombia root-knot nematode, Meloidogyne chitwoodi discovered in the State of Utah. Plant Disease 72: 363.
Hawkes, J.G., 1990. The Potato. Evolution, Biodiversity & Genetic Resources. Bethaven Press, London. 259 pp.
Hoyman, Wm.G., 1974. Reaction of Solanum tuberosum and Solanum species to Meloidogyne hapla. American Potato Journal 51: 281–286.
Hussey, R.S. & K.R., Barker, 1973. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Rep 57: 1025–1028.
Janssen, G.J.W., A.van, Norel, B., Verkerk-Bakker & R., Janssen, 1995. Detecting resistance to the root-knot nematodes Meloidogyne hapla and M. chitwoodi in potato and wild Solanum spp. Potato Res 38: 361–370.
Janssen, R., J., Bakker & F.J., Gommers, 1991. Mendelian proof for a gene-for-gene relationship between virulence of Globodera rostochiensis and the H1 resistance gene in Solanum tuberosum spp. andigena CPC 1673. Revué Nematologie 14: 213–219.
Johnston, S.A., T.P.M.den, Nijs, S.J., Peloquin & R.E., HannemanJr., 1980. The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet 57: 5–9.
Karssen, G., 1996. Description of M. fallax n.sp. (Nematoda: Heteroderidae), a root-knot nematode from the Netherlands. Fundamental and Applied Nematology (in press).
Matsubayashi, M., 1991. Phylogenetic relationships in the potato and its related species. In: T., Tsuchiya & P.K., Gupta (Eds.), Chromosome Engineering in Plants. Genetics, Breeding, Evolution, pp. 93–118. Elsevier, Amsterdam.
Meggelen, J.C.van, G., Karssen, G.J.W., Janssen, B., Verkerk-Bakker & R., Janssen, 1994. A new race of Meloidogyne chitwoodi Golden, O'Bannon, Santo & Finley, 1980? Fundamental and Applied Nematology 17: 93–96.
Novy, R.G. & R.E., HannemanJr., 1991. Hybridization between Gp. Tuberosum haploids and 1EBN wild potato species. American Potato Journal 68: 151–169.
Pinkerton, J.N. & G.A., McIntyre, 1987. The occurrence of Meloidogyne chitwoodi in potato fields in Colorado. Plant Disease 71: 192.
Roberts, P.A., 1992. Current status of the availability, development, and use of host plant resistance to nematodes. Journal of Nematology 24: 213–227.
Stone, A.R., 1985. Co-evolution of potato cyst nematodes and their hosts: implications for pathotypes and resistance. EPPO Bull 15: 131–137.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janssen, G.J.W., van Norel, A., Verkerk-Bakker, B. et al. Resistance to Meloidogyne chitwoodi, M. fallax and M. hapla in wild tuber-bearing Solanum spp.. Euphytica 92, 287–294 (1995). https://doi.org/10.1007/BF00037110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037110