ABSTRACT
Purpose
To investigate relationship between second virial coefficient B 2 and viscosity and aggregation propensity of highly concentrated monoclonal antibody (MAbs) solutions.
Methods
Intermolecular interactions of 3 MAbs solutions with varying pH were characterized according to B 2 estimated by analytical ultracentrifugation sedimentation equilibrium with initial loading concentrations <10 mg/mL. Viscosity measurements and stability assessments of MAb solutions at concentrations higher than 100 mg/mL were conducted.
Results
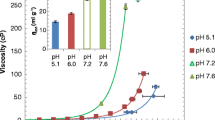
B 2 of all MAb solutions depended on solution pH and have qualitative correlation with viscosity and aggregation propensity. The more negative the B 2 values, the more viscous the solution, acquiring increased propensity to aggregate. Solutions with B 2 values of ~2 × 10−5 mL·mol/g2 acquire similar viscosity and aggregation propensity regardless of amino acid sequences; for solutions with negative B 2 values, viscosity and aggregation propensity differed depending on sequences. Results suggest attractive intermolecular interactions represented by negative B 2 values are influenced by surface properties of individual MAbs.
Conclusions
B 2 can be used, within certain limitations, as an effective indicator of viscosity and aggregation propensity of highly concentrated MAb solutions.









Similar content being viewed by others
REFERENCES
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23(9):1073–8.
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–402.
Treuheit MJ, Kosky AA, Brems DN. Inverse relationship of protein concentration and aggregation. Pharm Res. 2002;19(4):511–6.
Jiménez M, Rivas G, Minton AP. Quantitative characterization of weak self-association in concentrated solutions of immunoglobulin G via the measurement of sedimentation equilibrium and osmotic pressure. Biochemistry. 2007;46:8373–8.
Liu J, Nguyen MDH, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94(9):1928–40.
Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276(14):10577–80.
Minton AP. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J Pharm Sci. 2005;94(8):1668–75.
Harn N, Allan C, Oliver C, Middaugh CR. Highly concentrated monoclonal antibody solutions: direct analysis of physical structure and thermal stability. J Pharm Sci. 2007;96(3):532–46.
Kamerzell TJ, Kanai S, Liu J, Shire SJ, Wang YJ. Increasing IgG concentration modulates the conformational heterogeneity and bonding network that influence solution properties. J Phys Chem. 2009;113:6109–18.
Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99(3):1152–68.
Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab–Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97(10):4219–27.
Zhang J, Liu XY. Effect of protein-protein interactions on protein aggregation kinetics. J Chem Phys. 2003;119(20):10972–6.
Saluja A, Badkar AV, Zeng DL, Kalonia DS. Ultrasonic rheology of a monoclonal antibody (IgG2) solution: implications for physical stability of proteins in high concentration formulations. J Pharm Sci. 2007;96(12):3181–95.
Alford JR, Kwok SC, Roberts JN, Wuttke DS, Kendrick BS, Carpenter JF, et al. High concentration formulations of recombinant human Interleukin-1 receptor antagonist: I. Physical characterization. J Pharm Sci. 2008;97(8):3035–50.
Chari R, Jerath K, Badkar AV, Kalonia DS. Long- and short-range electrostatic interactions affect the rheology of highly concentrated antibody solutions. Pharm Res. 2009;26(12):2607–18.
Mahler HC, Friess W, Grauschopf U, Kiese S. Protein aggregation: pathways, induction factors and analysis. J Pharm Sci. 2009;98(9):2909–34.
Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–36.
Gokarn YR, Fesinmeyer RM, Saluja A, Cao S, Dankberg J, Goetze A, et al. Ion-specific modulation of protein interactions: anion-induced, reversible oligomerization of a fusion protein. Protein Sci. 2009;18(1):169–79.
Nishi H, Miyajima M, Nakagami H, Noda M, Uchiyama S, Fukui K. Phase separation of an IgG1 antibody solution under a low ionic strength condition. Pharm Res. 2010;27(7):1348–60.
Salinas BA, Sathish HA, Bishop SM, Harn N, Carpenter JF, Randolph TW. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J Pharm Sci. 2010;99(1):82–93.
Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23(8):643–51.
Nezlin R. Interactions between immunoglobulin G molecules. Immunol Lett. 2010;132(1–2):1–5.
Saluja A, Badkar AV, Zeng DL, Nema S, Kalonia DS. Ultrasonic storage modules as a novel parameter for analyzing protein-protein interactions in high protein concentration solutions: correlation with static and dynamic light scattering measurements. Biophys J. 2007;92:234–44.
Holde KE, Johnson C, Ho PS. Principles of physical biochemistry. Upper Saddle River: Pearson Education; 2006.
Neal BL, Asthagiri D, Lenhoff AM. Molecular origins of osmotic second virial coefficients of proteins. Biophys J. 1998;75:2469–77.
Attri AK, Minton AP. New methods for measuring macromolecular interactions in solution via static light scattering: basic methodology and application to nonassociating and self-associating proteins. Anal Biochem. 2005;337:103–10.
Alford JR, Kendrick BS, Carpenter JF, Randolph TW. Measurement of the second osmotic virial coefficient for protein solutions exhibiting monomer-dimer equilibrium. Anal Biochem. 2008;377:128–33.
Narayanan J, Liu XY. Protein interactions in undersaturated and supersaturated solutions: a study using light and X-ray scattering. Biophys J. 2003;84:523–32.
Brun VL, Friess W, Bassarab S, Mühlau S, Garidel P. A critical evaluation of self-interaction chromatography as a predictive tool for the assessment of protein-protein interactions in protein formulation development: a case study of a therapeutic monoclonal antibody. Eur J Pharm Biopharm. 2010;75(1):16–25.
Brun VL, Friess W, Bassarab S, Garidel P. Correlation of protein-protein interactions as assessed by affinity chromatography with colloidal protein stability: a case study with lysozyme. Pharm Dev Technol. 2010;15(4):421–30.
Harding SE, Rowe AJ, Horton JC. Analytical ultracentrifugation in biochemistry and polymer science. London: Royal Society of Chemistry; 1992. p. 90–125.
McGown EL, Hafeman DG. Multichannel pipettor performance verified by measuring pathlength of reagent dispensed into a microplate. Anal Biochem. 1998;258:155–7.
Sahin E, Grillo AO, Perkins MD, Roberts CJ. Comparative effects of pH and inonic strength on protein-protein intaractions, unfolding, and aggregation for IgG1 antibodies. J Pharm Sci. 2010;99(12):4830–48.
Engelsman J, Garidel P, Smulders R, Koll H, Smith B, Bassarab S, et al. Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm Res. 2011;28(4):920–33.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99(12):4812–29.
Frost RA, Caroline D. Diffusion of polystyrene in a theta mixed solvent (Benzene-2-Propanol) by Photon-correlation spectroscopy. Macromolecules. 1976;10(3):616–8.
Yamakawa H. Concentration dependence of the frictional coefficient of polymers in solution. 1962;36(11):2995–3001.
Lehermayr C, Mahler HC, Mäder K, Fischer S. Assessment of net charge and protein–protein interactions of different monoclonal antibodies. J Pharm Sci. 2011;100(7):2551–62.
Winzor DJ, Deszczynski M, Harding SE, Wills PR. Nonequivalence of second virial coefficients from sedimentation equilibrium and static light scattering studies of protein solutions. Biophys Chem. 2007;128:46–55.
Deszczynski M, Harding SE, Winzor DJ. Negative second virial coefficients as predictors of protein crystal growth: evidence from sedimentation equilibrium studies that refutes the designation of those light scattering parameters as osmotic virial coefficients. Biophys Chem. 2006;120:106–13.
Chi EY, Krishnan S, Kendrick BS, Chang BS, Carpenter JF, Randolph TW. Roles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor. Protein Sci. 2003;12:903–13.
Hawe A, Kasper JC, Friess W, Jiskoot W. Structural properties of monoclonal antibody aggregates induced by freeze-thawing and thermal stress. Eur J Pharm Sci. 2009;38:79–87.
ACKNOWLEDGMENTS & DISCLOSURES
The authors would like to thank Daisuke Ama, Kei Kubota, Yuki Araki, Mami Mitsui and Misako Sawakuri (Daiichi Sankyo Co., Ltd., Tokyo, Japan) for their skillful technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material Fig. 1
Residual plots generated from least-squares fitting of the concentration dependence of 1/M W,app for MAb-A (A), MAb-B(B), and MAb-C (C) in 10 mM citrate buffer containing 140 mM NaCl (pH 6). The data obtained for the entire concentration range 1 to 12 mg/mL were used for MAb-A and MAb-C. Data obtained at lower concentrations 1 to 3 mg/mL (□) and higher concentrations 4 to 12 mg/m (■) were used for MAb-B. (JPEG 33 kb)
Rights and permissions
About this article
Cite this article
Saito, S., Hasegawa, J., Kobayashi, N. et al. Behavior of Monoclonal Antibodies: Relation Between the Second Virial Coefficient (B 2) at Low Concentrations and Aggregation Propensity and Viscosity at High Concentrations. Pharm Res 29, 397–410 (2012). https://doi.org/10.1007/s11095-011-0563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0563-x