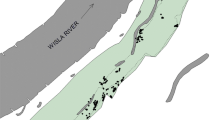
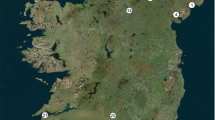
Abstract
Clonal plant species have been shown to adopt different strategies to persist in heterogeneous environments by changing relative investments in sexual reproduction and clonal propagation. As a result, clonal diversity and genetic variation may be different along environmental gradients. We examined the regional and local population structure of the clonal rhizomatous forest herb Paris quadrifolia in a complex of forest fragments in Voeren (Belgium). Relationships between population size (the number of shoots), shoot density (the number of shoots per m2) and local growth conditions were investigated for 47 populations. Clonal diversity and genetic variation within and among 19 populations were investigated using amplified fragment length polymorphism markers. To assess the importance of sexual reproduction, seed set, seed weight and germination success were determined in 18 populations. As predicted, local growth conditions largely affected population distribution, size and density of P. quadrifolia. Populations occurring in moist and relatively productive sites contained significantly more shoots. Here, shoots were also much more sparsely distributed compared to populations occurring in dry and relatively unproductive sites, where shoots showed a strongly aggregated distribution pattern. Clonal diversity was relatively high, compared with other clonal species (G/N ratio = 0.43 and Simpson’s D=0.81). Clonal diversity significantly (P<0.01) decreased with increasing shoot density while molecular genetic variation was significantly (P<0.01) affected by population size and local environmental conditions. Lack of recruitment and out-competition of less-adapted genotypes may explain the decreased genetic variation in dry sites. Analysis of molecular variance revealed significant genetic variation among populations (Φ ST=0.42, P<0.001), whereas pairwise genetic distances were not correlated to geographic distances, suggesting that gene flow among populations is limited. Finally, the number of generative shoots, the number of seeds per fruit and seed weight were significantly and positively related to population size and local growth conditions. We conclude that under stressful conditions populations of clonal forest plant species can slowly evolve into remnant populations characterized by low levels of genetic variation and limited sexual reproduction. Conservation of suitable habitat conditions is therefore a prerequisite for effective long-term conservation of clonal forest plant species.




Similar content being viewed by others
References
Auge H, Neuffer B, Erlinghagen F, Grupe R, Brandl R (2001) Demographic and random amplified polymorphic DNA analyses reveal high levels of genetic diversity in a clonal violet. Mol Ecol 10:1811–1819
Aspinwall N, Christian T (1992) Clonal structure, genotypic diversity, and seed production in populations of Filipendula rubra (Rosaceae) from the northcentral United States. Am J Bot 79:294–299
Cain ML, Damman H (1997) Clonal growth and ramet performance in the clonal herb, Asarum canadense. J Ecol 85:883–897
Cain ML, Dudle DA, Evans JP (1996) Spatial models of foraging in clonal plant species. Am J Bot 83:76–85
Damman H, Cain ML (1998) Population growth and viability analyses of the clonal woodland herb, Asarum canadense. J Ecol 86:13–26
de Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants: the foraging concept reconsidered. J Ecol 83:143–152
Diekmann M (2002) Species indicator values as an important tool in applied plant ecology: a review. Basic Appl Ecol 4:493–506
Dupré C, Ehrlén J (2002) Habitat configuration, species traits and plant distributions. J Ecol 90:796–805
Eckert CG (1999) Clonal plant research: proliferation, integration, but not much evolution. Am J Bot 86:1649–1654
Eckert CG, Barrett SCH (1993) Clonal reproduction and patterns of genotypic diversity in Decodon verticillatus (Lythraceae). Am J Bot 80:1175–1182
Ehrlén J, Eriksson O (2000) Dispersal limitation and patch occupancy in forest herbs. Ecology 81:1667–1674
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobot 18:1–248
Ellstrand NC, Roose ML (1987) Patterns of genotypic diversity in clonal plant species. Am J Bot 74:123–131
Eriksson O (1993) Dynamics of genets in clonal plants. Trends Ecol Evol 8:313–316
Eriksson O (1994) Stochastic population dynamics of clonal plants: Numerical experiments with ramet and genet models. Ecol Res 9:257–268
Eriksson O (1996) Regional dynamics of plants: a review of evidence for remnant, source-sink and metapopulations. Oikos 77:248–258
Fischer M, Matthies D (1998) RAPD variation in relation to population size and plant performance in the rare Gentianella germanica (Gentianaceae). Am J Bot 85:811–819
Fischer M, Husi R, Prati D, Peintinger M, van Kleunen M, Schmid B (2000) RAPD variation among and within small and large populations of the rare clonal plant Ranunculus reptans (Ranunculaceae). Am J Bot 87:1128–1137
Gardner SN, Mangel M (1999) Modeling investments in seeds, clonal offspring, and translocation in a clonal plant. Ecology 80:1202–1220
Giles BE, Goudet J (1997) Genetic differentiation in Silene dioica metapopulations: Estimation of spatiotemporal effects in a successional plant species. Am Nat 149:507–526
Hamrick JL, Godt MJW (1990) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding, and genetic resources. Sinauer, Sunderland, pp 43–63
Hegi G (1975) Illustrierte Flora von Mittel-Europa-5 Band: Dicotyledons. P Parey, Berlin
Honnay O, Verheyen K, Butaye J, Jacquemyn H, Bossuyt B, Hermy M (2002) Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecol Lett 5:525–530
Huff DR, Peakall R, Smouse PE (1993) RAPD variation within and among natural populations of outcrossing buffalograss (Buchloë dactyloides (Nutt) Engelm). Theor Appl Genet 86:927–934
Husband BC, Barrett SCH (1998) Spatial and temporal variation in population size of Eichhornia paniculata in ephemeral habitats: implications for metapopulation dynamics. J Ecol 86:1021–1031
Hutchings MJ, de Kroon H (1994) Foraging in plants: the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238
Jacquemyn H, Brys R, Hermy M (2002) Patch occupancy, population size and reproductive success of a forest herb (Primula elatior) in a fragmented landscape. Oecologia 130:617–625
Jacquemyn H, Butaye J, Hermy M (2003) Influence of environmental and spatial variables on regional distribution of forest plant species in a fragmented and changing landscape. Ecography 26:768–776
Jónsdóttir IS, Watson MA (1997) Extensive physiological integration: an adaptive trait in resource-poor environments. In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, pp 109–136
Kranczoch J (1997) Struktur and Dynamik in dichten Beständen von Paris quadrifolia (Trilliaceae). J Cramer, Berlin
Kudoh H, Shibaike H, Takasu H, Whigham DF, Kawano S (1999) Genet structure and determinants of clonal structure in a temperate deciduous woodland herb, Uvularia perfoliata. J Ecol 87:244–257
Lechowicz MJ, Bell G (1991) The ecology and genetics of fitness in forest plants 2 Microspatial heterogeneity of the edaphic environment. J Ecol 79:687–696
Lefort F, Douglas GC (1999) An efficient micro-method of DNA isolation from mature leaves of four hardwood tree species Acer, Fraxinus, Prunus and Quercus. Ann Sci 56:259–263
Lezberg AL, Halpern CB, Antos JA (2001) Clonal development of Maianthemum dilatatum in forests of differing age and structure. Can J Bot 79:1028–1038
Loehle C (1987) Partitioning of reproductive effort in clonal plants: a benefit-cost model. Oikos 49:199–208
Londo G (1976) Decimal scale for releves of permanent plots. Vegetatio 33:61–64
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
McCune B, Mefford MF (1999) Multivariate analysis of ecological data. PC-ORD Version 4.0 MjM Software, Gleneden Beach, Oregon
McLellan AJ, Prati D, Kaltz O, Schmid B (1997) Structure and analysis of phenotypic and genetic variation in clonal plants. In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, pp 185–210
Olivieri I, Michalakis I, Gouyon PH (1995) Metapopulation genetics and the evolution of dispersal. Am Nat 146:202–228
Peakall R, Smouse PE (2005) GenAlEx V6: genetic analysis in Excel. Population genetic software for teaching and research. Australian National University, Canberra, Australia. http://www.anu.edu.au/BoZo/GenAlEx/
Pielou EC (1969) An introduction to mathematical ecology. Wiley-Interscience, New York
Piquot Y, Petit D, Valero M, Cuguen J, de Laguerie P, Vernet P (1998) Variation in sexual and asexual reproduction among young and old populations of the perennial macrophyte Sparganium erectum. Oikos 82:139–148
Pornon A, Escaravage N, Thomas P, Taberlet P (2000) Dynamics of genotypic structure in clonal Rhododendron ferruginuem (Ericaceae) populations. Mol Ecol 9:1099–1111
Prati D, Schmid B (2000) Genetic differentiation of life-history traits within populations of the clonal plant Ranunculus reptans. Oikos 90:442–456
Roldán-Ruiz I, Dendauw J, Van Bockstaele E, Depicker A, De Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp). Mol Breed 6:125–134
Salisbury EJ (1942) Reproductive capacity of plants. Bell and Sons, London
Schaal BA, Leverich WJ (1996) Molecular variation in isolated plant populations. Plant Species Biol 11:33–40
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279
Soane ID, Watkinson AR (1979) Clonal variation in populations of Ranunculus repens. New Phytol 82:557–573
Stanton ML, Galen C (1997) Life on the edge: adaptation versus environmentally mediated gene flow in the snow buttercup, Ranunculus adoneus. Am Nat 150:143–178
Stanton ML, Galen C, Shore J (1997) Population structure along a steep environmental gradient: consequences of flowering time and habitat variation in the snow buttercup, Ranunculus adoneus. Evolution 51:79–94
Stehlik I, Holderegger R (2000) Spatial genetic structure and clonal diversity of Anemone nemorosa in late successional deciduous woodlands of Central Europe. J Ecol 88:424–435
Thompson JN (1999) Specific hypothesis on the geographic mosaic of coevolution. Am Nat 156:156–174
Thompson FL, Eckert CG (2004) Trade-offs between sexual and clonal reproduction in an aquatic plant: experimental manipulations vs phenotypic correlations. J Evol Biol 17:581–592
van Kleunen M, Fischer M, Schmid B (2001) Effects of intraspecific competition on size variation and reproductive allocation in a clonal plant. Oikos 94:515–524
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wade M, Goodnight CJ (1998) The theories of Fisher and Wright in the context of metapopulations: when nature does many small experiments. Evolution 52:1537–1553
Watkinson RA, Powell JC (1993) Seedling recruitment and the maintenance of clonal diversity in plant populations – a computer simulation of Ranunculus repens. J Ecol 81:707–717
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Acknowledgements
This study was funded by the Fund of Scientific Research (FWO). We are grateful to Alex Zeevaert for permission to perform this research and to Nancy Mergan for more than excellent assistance with the molecular analyses. Hans de Kroon and two anonymous referees provided useful comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jacqui Shykoff
Rights and permissions
About this article
Cite this article
Jacquemyn, H., Brys, R., Honnay, O. et al. Sexual reproduction, clonal diversity and genetic differentiation in patchily distributed populations of the temperate forest herb Paris quadrifolia (Trilliaceae). Oecologia 147, 434–444 (2006). https://doi.org/10.1007/s00442-005-0287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0287-x