Abstract
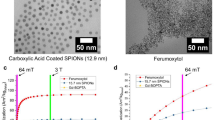
In this mini review, magnetic resonance imaging (MRI) contrast agents based on lanthanideoxide (Ln2O3) nanoparticles are described. Ln2O3 (Ln = Gd, Dy, Ho, and Er) nanoparticles are paramagnetic, but show appreciable magnetic moments at room temperature and even at ultrasmall particle diameters. Among Ln2O3 nanoparticles, Gd2O3 nanoparticles show larger longitudinal water proton relaxivity (r1) values than Gd-chelates because of the large amount of Gd in the nanoparticle, and the other Ln2O3 nanoparticles (Ln = Dy, Ho, and Er) show appreciable transverse water proton relaxivity (r2) values. Therefore, Gd2O3 nanoparticles are potential T1 MRI contrast agents while the other Ln2O3 nanoparticles are potential T2 MRI contrast agents at high MR fields.
Similar content being viewed by others
References
M. Rudin, Molecular imaging: principles and applications in biomedical research, 2nd Ed. (Imperial College Press, London, UK, 2005).
R. Weissleder and U. Mahmood, Radiology 219, 316 (2001).
J. C. Paeng and D. S. Lee, Open Nucl. Med. J. 2, 145 (2010).
T. F. Massoud and S. S. Gambhir, Genes & Development 17, 545 (2003).
R. H. Hashemi, W. G. Bradley and C. J. Lisanti, MRI The Basics, 2nd Ed. (Lippincott Williams & Wilkins, New York, 2004).
G. H. Lee, Y. Chang and T. J. Kim, Eur. J. Inorg. Chem. 2012, 1924 (2012).
W. Xu, K. Kattel, J. Y. Park, Y. Chang, T. J. Kim and G. H. Lee, Phys. Chem. Chem. Phys. 14, 12687 (2012).
T. J. Kim, K. S. Chae, Y. Chang and G. H. Lee, Curr. Topics Med. Chem. 13, 422 (2013).
H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnology 25, 1165 (2007).
R. B. Lauffer, Chem. Rev. 87, 901 (1987).
P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev. 99, 2293 (1999).
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, 2nd Ed. (Butterworth-Heinemann, New York, USA, 1997), p. 1243.
K. Kattel et al., ACS Appl. Mater. Interfaces 3, 3325 (2011).
K. Kattel et al., Biomaterials 33, 3254 (2012).
M. Norek, E. Kampert, U. Zeitler and J. A. Peters, J. Am. Chem. Soc. 130, 5335 (2008).
M. Norek, G. A. Pereira, C. F. G. C. Geraldes, A. Denkova, W. Zhou and J. A. Peters, J. Phys. Chem. C 111, 10240 (2007).
J. Y. Park et al., ACS Nano 3, 3663 (2009).
C. R. Kim, J. S. Baeck, Y. Chang, J. E. Bae, K. S. Chae and G. H. Lee, Phys. Chem. Chem. Phys. 16, 19866 (2014).
J. Fang, P. Chandrasekharan, X. Liu, Y. Yang, Y. Lv, C. Yang and J. Ding, Biomaterials 35, 1636 (2014).
H. T. Uyeda, I. L. Medintz, J. K. Jaiswal, S. M. Simon and H. Mattoussi, J. Am. Chem. Soc. 127, 3870 (2005).
J. Y. Park, K. Kattel, W. Xu, H. G. Kim, E. J. Kim, G. H. Lee, J. J. Lee, Y. Chang and T. J. Kim, J. Korean Phys. Soc. 59, 2376 (2011).
K. Kattel et al., J. Nanosci. Nanotechnol. 13, 7214 (2013).
C. Chang and D. Mao, Int. J. Chem. Kinet. 39, 75 (2007).
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 4th Ed. (Wiley-Interscience Publication, New York, 1980), p. 646, p. 984.
S. Arajs and R. V. Colvin, J. Appl. Phys. 35, 1181 (1964).
S. Arajs and R. V. Colvin, J. Appl. Phys. 33, 2517 (1962).
W. C. Koehler, E. O.Wollan and M. K.Wilkinson, Phys. Rev. 110, 37 (1958).
H. B. Lal, V. Pratap and A. Kumar, Pramana 10, 409 (1978).
R. M. Moon, W. C. Koehler, H. R. Child and L. J. Raubenheimer, Phys. Rev. 176, 722 (1968).
R. M. Moon and W. C. Koehler, Phys. Rev. B 11, 1609 (1975).
J. Blanusa, B. Antic, A. Kremenovic, A. S. Nikolic, L. Mazzerolles, S. Mentus and V. Spasojevic, Solid State Commun. 144, 310 (2007).
B. N. Figgis and J. Lewis, The Magnetochemistry of Complex Compounds,_In J. Lewis and R. G. Wilkins (Modern Coordination Chemistry, New York, Wiley, 1960), p. 406.
R. L. Carlin, Magnetochemistry (Springer-Verlag, Berlin, 1986), p. 238.
B. D. Cullity, Introduction to Magnetic Materials (Addison-Wesley Publishing Company, Reading, 1972), p. 102.
G. Wang, X. Zhang, A. Skallberg, Y. Liu, Z. Hu, X. Mei and K. Uvdal, Nanoscale 6, 2953 (2014).
E. S. Choi, J. Y. Park, K. Kattel, W. Xu, G. H. Lee, M. J. Baek, J. H. Kim, Y. Chang and T. J. Kim, J. Korean Phys. Soc. 56, 1532 (2010).
A. Roch, R. N. Muller and P. Gillis, J. Chem. Phys. 110, 5403 (1999).
P. Reimer and T. Balzer, Eur. Radiol. 13, 1266 (2003).
C. W. Jung and P. Jacobs, Magn. Reson. Imaging 13, 661 (1995).
J. Lodhia, G. Mandarano, N. J. Ferris, P. Eu and S. F. Cowell, Biomed. Imaging Interv. J. 6, e12 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, G.H., Chang, Y. Magnetic properties, water proton relaxivities, and in-vivo MR images of paramagnetic nanoparticles. Journal of the Korean Physical Society 67, 44–51 (2015). https://doi.org/10.3938/jkps.67.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3938/jkps.67.44