Abstract
Cardiac measures such as heart rate measurements are important indicators of both physiological and psychological states. However, despite their extraordinary potential, their use is restricted in comparative psychology because traditionally cardiac measures involved the attachment of sensors to the participant’s body, which, in the case of undomesticated animals such as nonhuman primates, is usually only possible during anesthesia or after extensive training. Here, we validate and apply a camera-based system that enables contact-free detection of animals’ heart rates. The system automatically detects and estimates the cardiac signals from cyclic change in the hue of the facial area of a chimpanzee. In Study 1, we recorded the heart rate of chimpanzees using the new technology, while simultaneously measuring heart rate using classic PPG (photoplethysmography) finger sensors. We found that both methods were in good agreement. In Study 2, we applied our new method to measure chimpanzees’ heart rate in response to seeing different types of video scenes (groupmates in an agonistic interaction, conspecific strangers feeding, nature videos, etc.). Heart rates changed during video presentation, depending on the video content: Agonistic interactions and conspecific strangers feeding lead to accelerated heart rate relative to baseline, indicating increased emotional arousal. Nature videos lead to decelerated heart rate relative to baseline, indicating a relaxing effect or heightened attention caused by these stimuli. Our results show that the new contact-free technology can reliably assess the heart rate of unrestrained chimpanzees, and most likely other primates. Furthermore, our technique opens up new avenues of research within comparative psychology and facilitates the health management of captive individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac signals are widely used as one of the main physical health parameters of both human and nonhuman individuals (e.g., Aiello, 2016; Hajar, 2018; Jensen, 2019). However, heart rate also plays a crucial role in psychological research. Already in the middle of the last century, it was found that emotionally arousing stimuli lead to measurable changes in heart rate in human adults (Darrow, 1929; Graham & Clifton, 1966; Lacey, 1959). That is, a participant’s heart rate can be indicative of their psychological activity. Especially in developmental psychology, measuring heart rate has proven an extremely useful tool to implicitly assess psychological activity in young participants who cannot yet verbally articulate their mental states or demonstrate complex behaviors (see Reynolds & Richards, 2008, for a review). Early research found, for example, that newborn infants’ heart rate accelerated when they experienced tactile or auditory stimulation (Davis et al., 1965). By contrast, heart rate decelerated as part of the orienting response, i.e., when infants focused their attention on a novel stimulus (e.g., Graham et al., 1983; Graham & Jackson, 1970). Indeed, heart rate can be used as an indicator for different phases of attention and information processing in infants. It has therefore been utilized in studies testing recognition memory, i.e., studies that tried to identify whether an infant perceived a stimulus as novel or as the same as a previously presented one. This has been done in the context of object categorization (Elsner et al., 2006), as well as in the context of numerical cognition (Brez & Colombo, 2012). Moreover, changes in heart rate have been used as a measure of positive affect in infants (Brock et al., 1986) and as a measure of infants’ ability to recognize the incongruity of absurd scenes in the context of humor perception (Mireault et al., 2018). In sum, measuring heart rate provides a fruitful way to assess both emotional states and cognitive processes in pre-verbal infants.
Measuring psychological activity in an implicit, non-verbal way is not only valuable for pre-verbal but even more so for non-verbal creatures. So far, however, only very few studies have implemented cardiac measures in cognitive research with animals, especially with humans’ closest living relatives, the nonhuman primates. These studies have mainly focused on heart rate as a measure of affective state. For chimpanzees (Pan troglodytes), for example, it was found that heart rate changes in response to hearing emotionally arousing auditory stimuli (Berntson et al., 1989) or viewing emotionally arousing pictures [(Boysen & Berntson, 1986; Boysen & Berntson, 1989); also see (Kano et al., 2016)]. Aureli et al. (1999) used heart rate as an indicator of emotional state during social interactions in rhesus macaques.
The relative dearth of studies using cardiac measures in comparative research is due to crucial practical limitations: until recently, measuring cardiac signals required one or several sensors to be attached to the participant’s body. In the case of nonhuman primates, this constitutes a severe constraint. This limitation implied that animals had to be trained extensively to tolerate the attachment of sensors, (e.g., Berntson et al., 1989; Boysen & Berntson, 1986; Boysen & Berntson, 1989; Kano et al., 2016; but see Cloutier Barbour et al., 2020), limiting the number of testable individuals to only a handful of animals worldwide. Alternatively, the attachment of sensors involved the animal being anesthetized (for sensors to be implanted, e.g., Aureli et al., 1999; Bliss-Moreau et al., 2013) or a jacket-system to be fixated (Derakhchan et al., 2014; Kremer et al., 2011) which, if done for the sole purpose of conducting the study, raises ethical concerns and excludes the specially protected great ape species. Additionally, there is evidence that exposure to anesthesia can have short- and long-term influences on cognitive performance both in humans (Caza et al., 2008; Chen et al., 2001; Ing et al., 2012; Rasmussen, 2006) and nonhuman primates (Raper et al., 2015; Talpos et al., 2019; Walters et al., 2019). Results obtained in cognitive tasks following anesthesia thus need to be interpreted with caution. Hence, the past requirement for physical contact of sensors with the participant’s skin has tremendously limited the applicability of cardiac measures in comparative psychological research.
This limited applicability is especially unfortunate since cardiac measures have the potential to provide a unique window into the emotional and cognitive world of nonhuman primates and other species. That is, heart rate signals could be operationalized to measure attentional and affective states in animals. For example, they could function as indicators of primates’ feelings in certain situations or in response to different stimuli. Also, they could complement behavioral measures such as looking-time in studies creating expectation violations to shed light on how nonhuman primates and other animals expect certain social or physical scenes to proceed [(Bräuer & Call, 2011; Horschler et al., 2019; Santos et al., 2005; Uller et al., 1997), for a review see (Winters et al., 2015)]. As with human infants, heart rate measures could also be used to test recognition memory, and therefore help reveal unobservable mental processes in various contexts. This would not only complement existing efforts to understand the evolution of cognition but would also enable cognitive testing of populations and groups of individuals that otherwise do not engage in classic cognitive tasks, such as very young or untrained individuals. Thus, making the measurement of cardiac signals in animals contact-free and with that more applicable would be highly beneficial for comparative psychology advancement.
Developing non-invasive, contact-free ways of measuring cardiac signals will not only open up new ways to get insights into the psychological processes of nonhuman primates and other animals but would also greatly enhance the health management of captive animals. Cardiovascular disease is among the main causes of mortality in captive great apes (Murphy et al., 2018). One of the reasons for this is that monitoring cardiac signals of these potentially dangerous animals is inherently difficult and usually only possible during anesthesia. As a result, signs of cardiac disease, such as abnormal heart rhythms (Lowenstine et al., 2016), are often only recognized very late when the disease has already progressed. The possibility to monitor the cardiac signals of great apes in a new, contact-free way, on a regular basis (perhaps as part of a daily routine), would help to recognize potential signs of cardiac disease earlier and might, therefore, help to lower great ape mortality linked to cardiac disease.
In recent years, the first attempts were made to monitor cardiac signals of nonhuman primates in a contact-free way. Unakafov et al. (2018) designed a non-contact pulse-monitoring system for rhesus macaques. Heart rate was measured by extracting the imaging photoplethysmography (iPPG) signal from a RGB (red, green, and blue) facial video. Although iPPG technology is increasingly used in remote physiological parameter detection for humans, it is limited in estimating cardiac signals for animals with intensely hair-covered skin or thicker epidermal layers. Moreover, the monkeys were restrained and head-stabilized during the study, limiting the value of the method for non-invasive cardiac signal assessment; also see (Froesel, 2020) for a recent study using contact-free HR estimation based on RGB videos and infrared videos with head-restrained rhesus macaques. In a recent pilot study (Al-Naji et al., 2019), a camera-based long-distance monitoring system was explored for extracting the cardiopulmonary signal (heart rate and breathing rate) of diverse exotic animals. The technique relied on the image motion-based method (Al-Naji, Gibson, et al., 2017a; Al-Naji, Gibson, et al., 2017b) rather than the skin color variation used by iPPG technology (Ming-Zher Poh, 2010; Wim Verkruysse, 2008). The motion-based method extracts the cardiopulmonary signal by exploiting the cyclic motion of the animal’s body due to the cardiopulmonary activity. The cardiopulmonary signal was extracted successfully from unrestrained and awake animals, including primates like the Sumatran orangutan (Pongo abelii) and hamadryas baboon (Papio hamadryas). This technique can be applied on complex surface textures to estimate pulse rate, and it may thus be useful for nonhuman primate studies. While the results of this study appeared extremely promising, they still lack validation by conventional methods.
Here, we demonstrate a non-contact, non-invasive, and real-time monitoring system to extract the heart rate of one of the most commonly tested nonhuman primate species in comparative psychological research, which is also amongst the great ape species most affected by cardiovascular disease: chimpanzees (Pan paniscus). In the first study, we validate our monitoring system by means of PPG sensors that were attached to the pointing finger of trained chimpanzees. In a second study, we demonstrate an application of the method as a means to assess the emotional reaction of chimpanzees to different stimuli.
Study 1
In this study, we validated our monitoring system by means of PPG sensors that were attached to the index finger of trained chimpanzees.
Methods
Animal and ethical considerations
The project was conducted at the Wolfgang Koehler Primate Research Center (WKPRC) in Leipzig, Germany. Chimpanzees were tested individually in their sleeping rooms. The chimpanzees were never food or water-deprived and participated in the study on a voluntary basis. The study was approved by the ethics committee of the Max Planck Institute for Evolutionary Anthropology and Leipzig Zoo. No medical, toxicological, or neurobiological research of any kind is conducted at the WKPRC. All research strictly adheres to the legal requirements of Germany. Animal husbandry and research at the WKPRC comply with the “EAZA Minimum Standards for the Accommodation and Care of Animals in Zoos and Aquaria”, the “WAZA Ethical Guidelines for the Conduct of Research on Animals by Zoos and Aquariums” and the “Guidelines for the Treatment of Animals in Behavioral Research and Teaching” of the Association for the Study of Animal Behavior (ASAB). Prior to data collection, all individuals were trained to extend their index finger through a small hole in the panel and be comfortable with the caregivers attaching a clip to the fingertip.
Setup and data acquisition
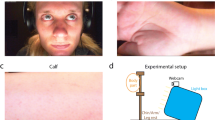
The study was performed in an indoor environment (the chimpanzees’ sleeping room), where a digital camera (Panasonic HC-V757 24.0 Mega Pixels) on a tripod was placed at a distance of between 0.5 and 1 m from the front of the chimpanzees’ enclosure, as shown in Fig. 1. To reduce chimpanzee movement, the videos were recorded while the chimpanzees were drinking juice out of a custom-made juice dispenser. At the same time, the chimpanzees extended their fingers through the hole in the Plexiglas to measure the heartbeat by a finger contact PPG sensor (BIOPAC MP160 with PPGED, Pulse range, 30–250 bpm) as the reference heart rate.
Seven chimpanzees (Pan troglodytes verus) participated in the study (Table 1). Each testing session lasted up to 10 min, depending on the willingness of the chimpanzee to sit still. Each chimpanzee was tested for up to two sessions, depending on their availability and willingness to participate. For illumination, we used artificial light during video recordings and there was no daylight in the sleeping rooms. The video data were captured with a resolution of 1280 × 720 and a frame rate of 25 fps, saved in MP4 format.
System framework and signal processing
The flowchart of the proposed system to compute cardiac signal from videos is presented in Fig. 2. First, we fed the video frame by frame into the system for processing. Once the first frame was captured, the ROI for extracting cardiac signal was manually selected as a bounding-box. Due to the strong reflections caused by the light on the Plexiglas panel (see the areas outlined with the orange dashed line in Fig. 3), we chose the facial area to be the ROI to avoid the noise from reflections (Fig. 2 ).
Cardiopulmonary activity can be extracted from subtle motion information of the body, which is caused by the mechanical flow of blood and the respiratory system (Al-Naji et al. 2017c). These subtle movements directly cause the reflected intensity change in the video. Accordingly, in the second step, since the videos were captured in RGB color space, the intensity information needed to be separated from the color information. The RGB color space was converted to YCbCr color space using the color space transformation matrix:
R, G, B refer to the pixel values from the RGB color space. We split the YCbCr frames into three single-channel images and used the intensity channel information to compute the cardiac signal. Then we averaged the intensity pixel values of frame sequences from the ROI with:
where I(x, y, t) is the intensity pixel value at image location (x, y) at time t from recorded frames, and |ROI|is the size of the detected ROI that was rectangular.
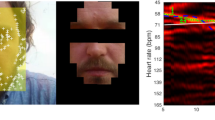
The third step is the most important process in the proposed system, which is signal processing. Breathing and cardiac activity both cause subtle body movements with oscillations of different frequencies. We use a band-pass filter (Butterworth coefficients (third-order)) to distinguish between the low-frequency breathing signal (0.25–0.8 Hz corresponding to 15–48 breaths per minute) and the high-frequency cardiac signal (1.5–4.2 Hz corresponding to 90–250 beats per minute). So, after bandpass filtering (1.5–4.2 Hz) of the raw signal extracted from the intensity channel, a complete ensemble empirical mode decomposition with adaptive noise (Colominas et al., 2014) was used to reduce noise interference caused by illumination variations from the raw signal. CEEMDAN decomposes the original signal into intrinsic mode functions (IMF s) with instantaneous amplitude and frequency data. An example of nine IMF decomposition of the Y signals after band-pass filter is provided in Fig. 4. The first mode (IMF 1) after separating the original signal was chosen for estimating the cardiac signal based on the frequency range that corresponds to the typical heartbeat range of chimpanzees (Kearns et al., 2000; Snyder, 1984; Weissler et al., 1961) as shown in Fig. 5.
In the fourth step of the process, a peak detection method was carried out to identify the periodicity of peaks, their locations, and number of peaks of the acquired signals. We computed the heart rate using the following equation,
where p is the number of peaks in the acquired signal, n is the number of frames of the selected video segment, and Fr is the frame rate.
Image processing for ROI stabilization
To reduce false positives of ROI due to target position change and movement during detection, we provide a stabilization method which uses MOSSE Tracker (Bolme et al., 2010) to stabilize the target in every frame. The MOSSE tracker minimizes the sum of squared error between the actual output of the correlation and the desired output of the correlation. We chose it because of its speed. After drawing a bounding-box for tracking target, a MOSSE filter was initialized using the following formula,
where H∗ is a complex conjugate of the filter. Fi represents the pre-processed cropped template for training images in the Fourier domain for the ith frame of the video. Gi is the training output image in the Fourier domain for the ith frame of the video. \({F}_i^{\ast }\) is the complex conjugate of Fi. Element-wise multiplication is denoted by the symbol ⨀.
After initialization, the MOSSE filter updates the tracking target from the ith frame is computed as:
where η equals to 0.125 as the learning rate to let the filter perform best (Bolme et al., 2010).
Figure 6 is the comparison of using MOSSE Tracker to track ROI and without tracker for processing a video. During long-term monitoring, when the chimpanzee moved like in Fig. 6a, b. Figure 6b shows that one easily loses the target when a chimpanzee moves their head away at time t1 and t2 while the ROI (blue bounding-box) is not being tracked. Figure 6b depicts the same video sequence as in Fig. 6a but using the stabilization method. It is visible that the ROI (yellow bounding-box) is following the head rotation. The raw signals from these two processes are shown in Fig. 6c. It is obvious that the signal (yellow line) processed with the tracker performs better.
Results
In this section, we estimated the pulse rate from video based on the motion of the body surface and compared the motion-based pulse rate (video PR) with the reference pulse rate from the finger-contact pulse sensor (PPG sensor). The outcome of our method for measuring heart rate is in agreement with the ground truth heart rate from the pulse sensor. As an example, Fig. 7 shows the results of the video signal and PPG signal from participant Hope. The raw signal from the PPG sensor is shown in Fig. 7a, which is over a total duration of around 10 min (600 s). In the finger sensor raw signal data, it is apparent that there is a lot of noise caused by movement during the period and also probably due to the thick and intensely pigmented skin of chimpanzee fingers. Therefore, a relatively clean data segment (550–560 s) was chosen to compare with the video data. Figure 7b is the smoothed signal from the data segment and the frequency spectrum. Because the video and reference data were recorded synchronously, we selected the video data from the same time period (550–560 s). After band-pass filtering at 1.5 to 4.2 Hz, and separating the signal using CEEMDAN, the cardiac signal extracted from video and its frequency spectrum are shown in Fig. 7c. For the frequency domain of the reference data and the video data, the fundamental frequency shows very close values in pulse rate (1.7 Hz in frequency domain equal to 102 beats per minute and 1.8 Hz in frequency domain equal to 108 beats per minute, respectively). Figure 7d shows the time series of an obvious noise segment (time period 460–480 s in Fig. 7a) and its frequency domain. In Fig. 7d, the error peaks can be easily found in the time domain and the frequency spectrum shows an invalid pulse rate (0.5 Hz).
To validate the usefulness of the motion-based cardiac signal, several quality metrics for video heart rate and contact PPG sensor heart rate are shown in Table 2.
There are ten sessions with different ROIs (narrow face, upper face/forehead, and mouth) which depended on the posture and location of the chimpanzee in the recorded video (they were constantly in motion). In order to have a suitable sequence of video frames (long enough and without target loss) to compare with the contact PPG sensor data, we excluded the noisy parts of each session and computed analysis over noise-free parts only. In this paper, we use 10 s of video data to calculate an average heart rate, and for each session, it needs at least ten consecutive heart rate values to compare with the finger sensor data. Therefore, our criterion for a noise-free sequence to be included was that it was at least 100 s long, during which no target loss occurred. So, in Table 2, the time length of each session for testing is just around 2–3 min. We computed the average video heart rate and the ground truth values of every session. The results show that the average video heart rates are close to the reference data, with the average of all mean errors of 3.4%.
The quality of the PPG signal from the PPG sensor might be sub-optimal due to the fact that it is designed based on human skin and, as we explained above, the skin of a chimpanzee’s finger is much thicker than human skin and the texture is rougher as well. Because of this, it is plausible that in Table 2 the correlation values of some sessions (Session 1, 5, and 6) are just around 0.5 but the average value for the overall data (from video and finger contact PPG sensor) are very close. Also, in Fig. 8, which plots the heartbeat from video and finger clip (Sessions 1, 3, and 9), we can see that the finger clip seems to have performed worse, producing unrealistic data. Some very obvious error values have been circled out in a yellow dash line box (normally the heartbeat would not rise or fall rapidly in such a short period of time). The values of the video heartbeat in comparison are rather stable. Thus, due to the unstable performance of the finger clip data, we added the Kolmogorov–Smirnov (KS) test to evaluate the ability of the video cardiac signal to capture subtle heart rate variability (Balakrishnan, 2013). In addition, the final heart rate value is calculated by peak detection (see Eq. 3), so incorrect or missing peaks may produce spurious peak-to-peak intervals, which caused errors in the heart rate. Comparing the distributions of time between successive peaks for each signal might be a better way to validate our method. The KS-test p value in Table 2 presents the distribution comparison results; all testing sessions have been accounted for by only considering noise-free intervals with a length within 25% of the average detected pulse period. From the p value, we can see that all pairs of distributions are similar. We present four pairs of beat distributions finger clips and videos from Session 1, 5, 6, and 9 in Fig. 9 (an example for different beat distribution shapes: Sessions 1 and 6 show the flat distribution; Sessions 5 and 9 show the peakier distribution). Our method performed a more stable capture capability compared to the data from the finger sensor in all the time ranges.
Because of the unstable performance of the finger sensor on the chimpanzees’ fingers, we also tested our method on humans with the same PPG sensor. We took videos 90–150 s long from three participants and measured the heart rate every 10 s in the same way as with the chimpanzees above. Results are shown in Table 3. The heart rate values are stable from the PPG sensor and the Pearson correlations are round 0.8 to 0.9, which are better than for the chimps (see supplementary material Fig. S1–Fig. S3).
Discussion
Our results show that a novel technique using signals extracted from digital cameras is suitable for measuring heart rate from the faces of chimpanzees. Figure 8 shows that the heart rate accuracy of the technique and the PPG sensor data are not in perfect agreement. We believe that this is mostly due to the fact that the contact PPG sensor was not entirely suitable for chimpanzee skin, rather than due to inaccuracy of our technique.
In this study, the measured pulse rates were in the range of 100–200 beats per minute (BPM) for the seven chimpanzees, with average individual values between 141.5 and 169.3 BMP. These values are within the range of previously reported chimpanzee heart rates 79–240 BPM (Berntson et al., 1989; Boysen & Berntson, 1989; Erickson & Olsen, 1985; Hyeroba, 2011; Kearns et al., 2000; Snyder, 1984; Weissler et al., 1961). Due to the previous difficulty of measuring the heart rate of chimpanzees, the so-far-published heart rate data stem either from awake infant chimpanzees or from anesthetized adult chimpanzees. Expected heart rates of awake adult chimpanzees should range right in between those of anesthetized adult chimpanzees (which were rather low, e.g., 79–91 BPM in Erickson & Olsen, 1985) and awake infant chimpanzees (which were rather high, e.g., 105–240 in Weissler et al., 1961). Our reported average heart rates fulfill this expectation and are thus likely to represent a realistic heart rate range of awake adult chimpanzees. We calculated the heartbeat by counting peaks from every acquired signal. A time window of 10–30 s is required to estimate accurate results.
Since all of the videos were recorded during feeding to reduce movement, long-term observations where the chimpanzee is moving at will could raise some additional challenges. As an alternative means, pulse rate can be estimated from the peak-to-peak interval, which relies heavily on the quality of the signal but might require only a few seconds. In addition, from the p values in Table 2 and Fig. 9, it is apparent that our method performed better than the finger sensor in capturing beat-to-beat interval time, which means that the method has great potential to measure heart rate variability accurately. Future work will explore suitable algorithms to define the systolic and diastolic peaks in the extracted signal, and then detect the presence of the shape in the signal.
This study is the extension of research in “A Pilot Study for Estimating the Cardiopulmonary Signals of Diverse Exotic Animals Using a Digital Camera” (Al-Naji et al., 2019). Based on the previous method, the major improvements are: (1) we introduce CEEMDAN (Colominas et al., 2014) to reduce the illumination variations in signal processing part; (2) a stabilization method based on MOSSE Tracker has been used to reduce the noise caused by movements and also to avoid losing the target in the ROI; (3) for the goal of long-term monitoring, the system is running with the possibility of real-time heart rate detection; (4) we validated our method with controlled data from chimpanzees. The results suggest that our study should be suitable for all non-human primates.
Study 2
In the second study, we demonstrate an application of the method to assess chimpanzees’ emotional reactions to different video stimuli.
Methods and procedures
Animal and ethical considerations
The same seven individuals participated as in Study 1.
Setup and data acquisition
The setup was identical to Study 1, except that we did not use a PPG finger sensor. Additionally, we placed a screen (37.5 x 30 cm) in front of the Plexiglas panel on which the chimpanzees were presented with different video scenes. The screen was connected to a laptop and video presentations were managed using the software Teobii (Version 3.3.0). The chimpanzees saw three types of video scenes: In the aggression condition, chimpanzees saw conspecifics of their own social group engaged in an aggressive interaction. In the eating condition, the chimpanzees saw conspecifics of another social group (housed at the same zoo) eating large amounts of food. Finally, in the nature condition, the chimpanzees saw videos of natural habitats captured by a drone (see Fig. 10). Both the aggression and the eating scenes included naturally occurring sounds (e.g., screams in aggression scenes, background noises such as bird song in the eating scenes). The nature videos were accompanied by soft ambient music.
Example images of the three types of videos shown to the chimpanzees. In the aggression condition, familiar conspecifics were engaged in an aggressive interaction. In the eating condition, unfamiliar individuals consumed large amounts of food. In the nature condition, natural habitats were filmed from the bird’s perspective
Within one session, the chimpanzees were presented with one trial of each video category. In total, two sessions were administered. That is, the chimpanzees saw two example scenes of each video category (only one chimpanzee, Dorien, saw four examples in total. Her first two sessions were not analyzable due to technical errors and disturbance in the group, respectively). The order of conditions within each session was randomized. Also, the order of example scenes across sessions was randomized (e.g., approximately half of the individuals saw example scene A of the eating condition in their first session, the rest saw it in their second session).
Each video presentation was preceded by the presentation of a grey screen. During this grey-screen presentation, we measured the chimpanzees’ baseline heart rate prior to the video presentation (pre-base). The luminance of the grey screen was identical to that of a representative frame of the respective video. This was to avoid that the chimpanzees’ heart rate changed during video presentation simply due to its visual properties (i.e., its brightness). After the presentation of the video, chimpanzees saw again the same grey screen as before. Here, we recorded the chimpanzees’ heart rate as a post video baseline (post-base). Hence, each trial consisted of three phases: the pre-base, the video, and the post-base. Each of the phases lasted 10 s (see Fig. 11 for an illustration of a trial).
We expected the chimpanzees’ heart rates to change during video presentation relative to the pre-baseline, and to recover during the post-baseline. Further, we predicted the magnitude of change to be influenced by the content of the video: The highest magnitude of change was to be expected in the aggression condition because previous studies have demonstrated that viewing agonistic interactions leads to high levels of arousal in chimpanzees (e.g., Dezecache et al., 2017; Kano et al., 2016). Accordingly, we expected heart rate to increase in this condition. We also expected heart rate to increase during video presentation in the eating condition. This is because we assumed that viewing unfamiliar conspecifics is sufficiently arousing for highly territorial animals, who, in the wild, regularly engage in potentially lethal inter-group interactions (Boesch et al., 2008; Watts et al., 2006; Watts & Mitani, 2001; Wilson & Wrangham, 2003). Similarly, we expected the large amounts of food visible in the eating condition to be potentially arousing to the highly food-motivated animals. In comparison to the aggression condition, however, we expected the relative change in heart rate to be more subtle, mostly because the depicted scene involved fewer indications of potentially arousing affective states. More specifically, while depicted chimpanzees in the eating condition were calmly sitting together, the chimpanzees in the aggression condition uttered screams and showed facial expressions such as fear grins. We expected no change in heart rate during the nature condition because the depicted scenes are supposedly irrelevant to chimpanzees.
Data analysis
We measured the chimpanzees’ average heart rate during each of the observation phases from video, using the same approach as described for Study 1. Only those trials were included in the analysis where the average heart rate could be calculated for at least pre-base and video phase. Trials in which data for either of these two phases were missing (e.g., because the chimpanzee turned their head away), were excluded. In total, we analyzed 40 trials.
To test if the relative change of heart rate from pre-baseline to video presentation was different depending on which video category the ape had seen, we built a linear mixed model (Bates et al., 2015) with Gaussian error distribution. Average heart rate change (from pre-base to video) was our response variable. As fixed effects, we included condition (eating vs. aggression vs. nature) and session number (within subject, either 1, 2, 3, or 4). Subject was included as random effect on condition. Session was z-transformed (to a mean of 0 and a standard deviation of 1). To keep type 1 error rate to the nominal level of 5% (Barr, 2013; Schielzeth & Forstmeier, 2009), we included all possible random slopes components (condition and session number within subject) and the respective correlations between random slopes and intercepts. The significance of the full model as compared to the null model (comprising only session and the random effect subject) was established using a likelihood-ratio test (R function ANOVA with argument test set to ’Chisq’; (Dobson & Barnett, 2008; Forstmeier & Schielzeth, 2011)). P values for the individual effects were obtained using the package ’lmerTest’ (Kuznetsova et al., 2017).
To check if, within each of the three conditions, heart rate was different during the three trial phases, we ran three linear mixed models with Gaussian error distribution, one for each condition. Average heart rate was the response variable. Phase (pre-base, video, post-base), as well as (z-transformed) session were included as fixed effects. Subject was included as random effect on phase. We included all possible random slopes components (phase and session number within subject) and the respective correlations between random slopes and intercepts. The significance of the full model as compared to the null model (comprising only session and the random effect subject was established using a likelihood-ratio test (R function ANOVA with argument test set to “Chisq”; (Dobson & Barnett, 2008; Forstmeier & Schielzeth, 2011)). P values for the individual effects were based on likelihood-ratio tests comparing the full model with respective reduced models (R function drop1). All models were fitted in R (R Core Team, 2021) using the function lmer of the R-package lme4 (Bates et al., 2015). Raw data and analysis code can be found in the supplementary material.
Results
We found a highly significant effect of condition (i.e., video category) on the relative HR change from pre-baseline to video presentation (X2 = 9.91, df = 2, p = 0.002), i.e., heart rate changed differently depending on the video category. As visible in Fig. 12, there was a significant difference in HR change between the nature and the aggression condition (estimate ± SE = – 15.332 ± 3.771, df = 2, p = 0.001, CI (– 22.723, – 7.942)), with a decrease in heart rate in the nature condition, and an increase in heart rate in the aggression condition. There was also a significant difference in HR change between the nature and the eating condition (estimate ± SE = 12.786 ± 4.169, df = 2, p = 0.0134, CI (20.957, 4.614)), with an increase in heart rate in the eating condition. There was no difference between the eating and the aggression condition (estimate ± SE = – 2.547 ± 4.339, df = 2, p = 0.570, CI (– 11.051, 5.957)), but Fig. 12 illustrates that the heart rate change was slightly more pronounced in the aggression condition. There was no effect of session number (estimate ± SE = 0.454 ± 3.246; df = 1; p = 0.896).
Heart rate change during video presentation relative to the pre-baseline in the three conditions. Dots represent individual data per session for each chimpanzee. Dashed line represents pre-baseline level. Turquoise stars depict average changes over all individuals and sessions with bootstrapped 95% confidence intervals
Looking at the three conditions separately, we found a significant effect of phase on heart rate in the nature condition (X2= 7.436, df = 2, p = 0.01817). More specifically, heart rate decreased significantly during the video presentation compared to the pre-baseline (estimate ± SE= – 9.571 ± 2.695, df = 2, p = 0.009, CI (– 15.661, – 3.482)) and was also significantly lower than in the post-baseline (estimate ± SE = – 9.619 ± 4.14, df = 2, p = 0.047, CI ± (0.611, 18.973); see Fig. 12 and Fig S.7 in the supplementary material). There was no significant effect of phase in either the eating condition (X2 = 0.440, df = 2, p = 0.3818) or the aggression condition (X2 = 2.363, df = 2, p = 0.3432). Individual data for each session are visualized in the supplementary material (Fig. S 4–Fig. S 6).
Discussion
In this study, we measured the heart rate of chimpanzees in response to viewing different categories of social and non-social videos. We found that heart rate changed in response to the video playbacks. The direction and magnitude of change were dependent on the content of the video. As expected, heart rate increased during both the aggression condition and the eating condition (although absolute levels of heart rate were not significantly different from the pre- and post-baseline in both cases). While heart rate accelerated slightly more in the aggression condition than in the eating condition, this difference was not significant, suggesting that both feeding conspecifics from a foreign group and agonistic interactions of group mates had an arousing effect on chimpanzees. These findings align with previous results using different measures of emotional arousal (Dezecache et al., 2017; Kano et al., 2016).
Interestingly, and contrasting our predictions, we found a significant decrease in heart rate during the presentation of nature videos. There are several possible explanations for this finding.
The first possible explanation for the chimpanzees’ decelerated heart rate in the nature condition is that the nature stimuli may have induced the orienting response, i.e., an increase in attention with low levels of emotional arousal.
When human infants orient towards and attend to a (typically novel) stimulus, they enter a phase of “sustained attention” in which their heart rate is lower compared to baseline levels. Heart rate only resumes to baseline levels once the infant terminates attention (e.gColombo, 2001; Lansink, 1997; Richards, 1992). This particular change of heart rate is part of the so-called orienting response, which presumably subserves enhanced stimulus detection and processing (e.g., Graham & Clifton, 1966; Stekelenburg, 2002).
There is some evidence for a similar effect in chimpanzee infants (Berntson, 1984; Berntson, 1989; Berntson et al., 1989). In these studies, chimpanzee infants were presented with auditory stimuli, such as conspecific screams and conspecific laughter (Berntson et al., 1989), or threat, stress, and alarm vocalizations (Berntson, 1989) or with a combination of auditory and tactile stimuli (a tone and a vibration, Berntson, 1984). The infant chimpanzees’ heart rates decelerated when they listened to screams (Berntson et al., 1989) and, upon initial presentation, also when they listened to threat, stress, and alarm vocalizations (but upon repeated exposure, the authors report heart rate acceleration in response to the same stimuli, Berntson, 1989). Conversely, chimpanzee infants’ heart rate accelerated when they listened to conspecific laughter (Berntson et al., 1989), and upon first exposure to the tone and the tactile stimulus (but upon repeated exposure, the authors report heart rate deceleration in response to the same stimuli, Berntson, 1984). One study also assessed juvenile chimpanzees’ heart rates in response to visual stimuli (Boysen & Berntson, 1989). Here it was found that heart rate decelerated in response to seeing pictures of an unfamiliar chimpanzee, whereas it accelerated in response to seeing pictures of an aggressive chimpanzee. In sum, previous research found heart rate deceleration in response to hearing negatively valanced stimuli (aggression and stress) and seeing neutral foreign conspecifics.
In contrast to these previous studies, the chimpanzees’ heart rates in our study accelerated in response to the videos depicting aggressive interactions, which were also accompanied by threat vocalizations and screams (which is in accordance with other, more recent studies showing increased emotional arousal in response to such displays, e.g., Dezecache et al., 2017; Kano et al., 2016). Also, it increased in response to seeing unfamiliar conspecifics. These differences could be either due to the difference in modality (auditory or static picture stimuli in previous studies vs. video (visual + auditory) stimuli in our study), or to differences in the tested age class (infant and juvenile chimpanzees in previous studies vs. adult chimpanzees in our study), or to differences in the method used to measure heart rate. Currently, it remains unclear if adult chimpanzees show signatures of the orienting response when presented with dynamic visual stimuli and when heart rate is measured with state-of-the-art technology.
However, the finding that chimpanzees’ heart rate decelerated in the nature condition in our study could be interpreted as being indicative of the chimpanzees displaying the orienting response in this condition. Presumably, all our videos attracted the apes’ attention, which should, according to the theory of the orienting response, result in a temporarily decelerated heart rate. The videos of conspecifics, however, may have additionally induced emotional arousal, leading to overall higher heart rates compared to baseline levels. The videos of natural landscapes, by contrast, may have not induced emotional arousal but still induced sustained attention, leading to overall lower heart rates relative to baseline levels. It is noteworthy that both explanations for the decelerated heart rate in the nature condition are not mutually exclusive. It is well possible that the nature stimuli, in contrast to the conspecific stimuli, activated both the orienting response and had a calming effect on the chimpanzees.
Future research should use a method with higher temporal resolution to investigate if the typical heart rate changes associated with the orienting response can be found in adult chimpanzees and under which conditions stimuli induce the orienting response rather than emotional arousal/relaxation.
A second possible explanation for the decelerated heart rate in the nature condition is that viewing these stimuli had a relaxing effect on chimpanzees. Such a calming effect might have been caused by the perceptual features of these stimuli. More specifically, the nature scenes involved slowly and regularly moving patterns, whereas the other scenes, especially the aggression scene, involved irregularly and fast-moving elements. Moreover, in contrast to the two other conditions, the nature scenes were accompanied by soft, ambient music. This type of music is known to have a relaxing effect on humans (e.g., Bernardi et al., 2006; Chlan, 1998; Hilz et al., 2014; Krumhansl, 1997; Nomura et al., 2013) and may hence also cause a decrease in heart rate in other apes. It is possible that either the ambient music, or the perceptual properties of the nature scene, or a combination of both, had a relaxing effect on the chimpanzees, irrespective of the depicted content.
Alternatively, the content of the stimuli, i.e., the depicted nature scenes themselves, may have had a relaxing effect on the apes. This would be unexpected because the video scenes were drone footage of different natural habitats shot from a bird’s perspective. That is, they depicted views entirely unfamiliar to our chimpanzees.
Studies on humans have found that spending time in nature, or viewing nature-related stimuli, does have a relaxing effect on humans, indicated, among others, by decelerated heart rate (Brown et al., 2013; Park et al., 2010; Sahlin et al., 2016). The finding that our closest living relatives may react similarly to natural stimuli they have never encountered in real life could suggest that this relaxing effect of nature does not depend on previous positive experiences in the depicted habitats. Instead, the positive, calm feeling many humans associate with spending time in nature may be grounded in an innate physiological response to natural environments deeply rooted in our evolutionary history. Natural environments may be important for our physiological and mental well-being, and the finding that this importance might have an evolutionary origin could emphasize the value of nature conservation. Assuming that lower resting heart rate is associated with lower stress levels and better well-being (e.g., Aureli et al., 1999; Sommerfeldt, 2019; Shively, 2015), our results also suggest that cardiac measures might be a promising tool for assessing the welfare of captive chimpanzees and other animals.
When designing enclosures, zoos typically try to ensure that they are suitable for animals to fulfill their primary biological needs and, more recently, naturalistic designs are often favored over artificial optics. One main motivation behind this trend is that visitors value naturalistic enclosures and enrichment over non-naturalistic ones (e.g., Davey, 2006; Kutska, 2009; Melfi et al., 2004); Ogden et al., 1993; Wolf & Tymitz, 1981). Some studies also investigated the benefits of naturalistic enclosures for animal welfare and found that these designs are often associated with the expression of natural behaviors and low frequencies of psychopathologies (e.g., Chang et al., 1999; Fàbregas et al., 2012; Maple & Finlay, 1986; Maple & Stine, 1982). Other studies, however, suggested that it is not a naturalistic appearance that is crucial for animal welfare, but other factors like sufficient space or functional enclosure complexity (e.g., Cassinello & Pieters, 2000; Clubb & Mason, 2003; Clubb & Mason, 2007; Fàbregas et al., 2012; Stroud, 2007). In fact, some authors suggested that the naturalistic appearance of an enclosure is entirely unsuitable for assessing how well it meets the needs of its occupants (Melfi et al., 2004; Shepherdson, 1998). While more systematic research is needed to draw firm conclusions, our study may give a first hint that viewing nature-related stimuli might have positive effects on the well-being of chimpanzees (and possibly other animals). We suggest that future studies should complement traditional welfare measures such as ranking the degree of expressed natural behaviors and psychopathologies (see, e.g., Maple & Perdue, 2013 for a comprehensive review of traditional measures of well-being and well-fare of zoo animals) with contact-free cardiac measures in order to assess an animals’ well-being in a more nuanced and direct manner. Importantly, once the appropriate algorithms have been developed, future research should also consider an animal’s heart rate variability (HRV), i.e., the variability of the time intervals between consecutive heartbeats. HRV is indicative of the functioning of the autonomic nervous system, in particular between sympathetic and vagal activity, and therefore gives a more comprehensive and detailed picture of an individual’s physiological and psychological state compared to heart rate alone (e.g., McCraty, 1995; Sleigh & Henderson, 1995). HRV is regularly used as a marker of acute and chronic stress in humans (Delaney & Brodie, 2000; Hall et al., 2004). Also in animals, HRV is often assessed to analyze changes in sympathovagal balance related to diseases, psychological and environmental stressors, and welfare (see Von Borell, 2007 for a review). Ideally, future studies should systematically assess and compare the resting heart rate and HRV in individuals of the same species living in different types of enclosures that vary with regard to their level of natural appearance. If such studies confirmed that animals living in more natural enclosures have on average lower resting heart rates and higher HRV, this would suggest that both functional complexity and natural appearance should be considered when designing new enclosures.
General discussion
In this study, we validated a new method of measuring heart rate in a contact-free way in a nonhuman species. The results show that our technique can estimate the heart rate of conscious and unrestrained chimpanzees without special hardware for illumination. Furthermore, since the input videos can be recorded with a standard digital camera, our technique is affordable and easy to use. It thus has the potential to be applicable in a wide range of contexts and setups.
In addition to validating our method, we have also demonstrated a simple application for comparative psychological research. We obtained findings that have potential implications for the evolutionary origins of the positive effects of nature on human well-being, and for the welfare of zoo-housed apes and other animals. This shows that our technique opens up new routes to study aspects of animals’ emotional and cognitive worlds that have previously been obscured. In the current study, we used contact-free heart rate monitoring to measure emotional arousal in chimpanzees. For future research, it will be interesting to try and measure more subtle changes of emotional states, such as surprise or changes in cognitive processing such as cognitive load. Being able to monitor these states will open up an even wider range of possible applications and research questions. Measuring heart rate in a contact-free way brings many advantages in comparison to traditional techniques. First, it increases the number of animals for psychological research with nonhuman primates. Standard measures that involved attaching one or several sensors to a participant’s body were only applicable with very well trained individuals or individuals who underwent anesthesia before the studies. This constraint severely limited the number of testable individuals, especially for research with great apes. Our technique eliminates these constraints and hence allows for much larger samples which, in turn, lead to more meaningful results of comparative studies. In addition, the contact-free nature of our technique also allows observing groups of individuals who do not readily interact with researchers and hence have rarely been tested in comparative research. For example, young great ape infants who are still clinging to their mothers have been practically inaccessible to psychological research, leaving significant gaps in knowledge regarding their cognitive development. Our technique makes this group of individuals accessible for cognitive testing and allows us to ask new research questions in the area of comparative developmental research.
The second advantage of contact-free heart rate monitoring is that it has the potential to improve the health management of captive animals. More specifically, the contact-free and easy application of the method allows for regular monitoring of animals’ cardiac signals without disturbing the daily routine of the animals and their caretakers. In turn, this may facilitate recognizing potential signs of cardiac disease in the early stages and thereby help lower mortality linked to cardiac disease. Hence, our method is not only beneficial for basic cognitive research but can also greatly enhance the health management of a wide range of animal species.
In order to allow for unattended monitoring of great apes’ heart rate, future research should employ more complex filtering and decomposition algorithms to enhance the robustness of the pulse signal extraction. More specifically, our current method is limited to situations in which animals remain relatively still for at least 5–6 consecutive seconds, such as during feeding or sleeping. Large or sudden movements will cause the tracker to lose the target and signal extraction will be interrupted. More complex filtering and decomposition algorithms will be needed to reduce susceptibility to movement and make it more applicable for a wider range of contexts.
References
Aiello, S. E. (2016). The Merck veterinary manual (p. 3325). White Station, NJ, USA: Merck & Company, Incorporated.
Al-Naji, A., Gibson, K., Lee, S.-H., & Chahl, J. (2017a). Monitoring of cardiorespiratory signal: Principles of remote measurements and review of methods. IEEE Access, 5, 15776–15790.
Al-Naji, A., Gibson, K., Lee, S.-H., & Chahl, J. (2017b). Real-time apnoea monitoring of children using the Microsoft Kinect sensor: A pilot study. Sensors, 17, 286.
Al-Naji, A., Perera, A., & Chahl, J. (2017c). Remote monitoring of cardiorespiratory signals from a hovering unmanned aerial vehicle. Biomedical Engineering Online (16), 101.
Al-Naji, A., Tao, Y., Smith, I., & Chahl, J. (2019). A pilot study for estimating the cardiopulmonary signals of diverse exotic animals using a digital camera. Sensors, 19, 5445.
Aureli, F., Preston, S. D., & de Waal, F. (1999). Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): A pilot study. Journal of Comparative Psychology, 113, 59.
Balakrishnan, G. D. F. (2013). Detecting pulse from head motions in video[C]. Proceedings of the IEEE conference on computer vision and pattern recognition., 3430–3437.
Barr, D. (2013). Random effects structure for testing mixed-effects models. Frontiers in Psychology, 4, 328.
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, Articles, 67, 1–48. https://doi.org/10.18637/jss.v067.i01
Bernardi, L., Porta, C., & Sleight, P. (2006). Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: The importance of silence. Heart, 92, 445–452.
Berntson, G. G. (1984). Cardiac startle and orienting responses in the great apes. Behavioral Neuroscience, 98(5), 914–918. https://doi.org/10.1037/0735-7044.98.5.914
Berntson, G. G. (1989). Specificity of the cardiac response to conspecific vocalizations in the chimpanzee. Behavioral Neuroscience, 103, 235–245.
Berntson, G. G., Boysen, S. T., Bauer, H. R., & Torello, M. S. (1989). Conspecific screams and laughter: Cardiac and behavioral reactions of infant chimpanzees. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 22, 771–787.
Bliss-Moreau, E., Machado, C. J., & Amaral, D. G. (2013). Macaque cardiac physiology is sensitive to the valence of passively viewed sensory stimuli. PLoS One, 8, e71170.
Boesch, C., Crockford, C., Herbinger, I., Wittig, R., Moebius, Y., & Normand, E. (2008). Intergroup conflicts among chimpanzees in Taı̈ National Park: Lethal violence and the female perspective. American Journal of Primatology: Official Journal of the American Society of Primatologists, 70, 519–532.
Bolme, D. S., Beveridge, J. R., Draper, B. A., & Lui, Y. M. (2010). Visual object tracking using adaptive correlation filters. 2010 IEEE computer society conference on computer vision and pattern recognition, (pp. 2544–2550).
Boysen, S. T., & Berntson, G. G. (1986). Cardiac correlates of individual recognition in the chimpanzee (pan troglodytes). Journal of Comparative Psychology, 100, 321.
Boysen, S. T., & Berntson, G. G. (1989). Conspecific recognition in the chimpanzee (pan troglodytes): Cardiac responses to significant others. Journal of Comparative Psychology, 103, 215.
Bräuer, J., & Call, J. (2011). The magic cup: Great apes and domestic dogs (Canis familiaris) individuate objects according to their properties. Journal of Comparative Psychology, 125, 353.
Brez, C. C., & Colombo, J. (2012). Your eyes say “no,” but your heart says “yes”: Behavioral and psychophysiological indices in infant quantitative processing. Infancy, 17, 445–454.
Brock, S. E., Rothbart, M. K., & Derryberry, D. (1986). Heart-rate deceleration and smiling in 3-month-old infants. Infant Behavior and Development, 9, 403–414.
Brown, D. K., Barton, J. L., & Gladwell, V. F. (2013). Viewing nature scenes positively affects recovery of autonomic function following acute-mental stress. Environmental Science & Technology, 47, 5562–5569.
Cassinello, J., & Pieters, I. (2000). Multi-male captive groups of endangered dama gazelle: Social rank, aggression, and enclosure effects. Zoo Biology: Published in affiliation with the American Zoo and Aquarium Association, 19, 121–129.
Caza, N., Taha, R., Qi, Y., & Blaise, G. (2008). The effects of surgery and anesthesia on memory and cognition. Progress in Brain Research, 169, 409–422.
Chang, T. R., Forthman, D. L., & Maple, T. L. (1999). Comparison of confined mandrill (Mandrillus sphinx) behavior in traditional and “ecologically representative” exhibits. Zoo Biology: Published in affiliation with the American Zoo and Aquarium Association, 18, 163–176.
Chen, X., Zhao, M., White, P. F., Li, S., Tang, J., Wender, R. H., . . . others. (2001). The recovery of cognitive function after general anesthesia in elderly patients: A comparison of desflurane and sevoflurane. Anesthesia & Analgesia, 93, 1489–1494.
Chlan, L. (1998). Effectiveness of a music therapy intervention on relaxation and anxiety for patients receiving ventilatory assistance. Heart & Lung, 27, 169–176.
Cloutier Barbour, C., Danforth, M. D., Murphy, H., Sleeper, M. M., & Kutinsky, I. (2020). Monitoring great ape heart health through innovative electrocardiogram technology: Training methodologies and welfare implications. Zoo Biology, 39, 443–447.
Clubb, R., & Mason, G. (2003). Captivity effects on wide-ranging carnivores. Nature, 425, 473–474.
Clubb, R., & Mason, G. J. (2007). Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Applied Animal Behaviour Science, 102, 303–328.
Colombo, J. R. (2001). Heart rate defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Development, 72(6), 1605–1616.
Colominas, M. A., Schlotthauer, G., & Torres, M. E. (2014). Improved complete ensemble EMD: A suitable tool for biomedical signal processing. Biomedical Signal Processing and Control, 14, 19–29.
Darrow, C. W. (1929). Differences in the physiological reactions to sensory and ideational stimuli. Psychological Bulletin, 26, 185.
Davey, G. (2006). Relationships between exhibit naturalism, animal visibility and visitor interest in a Chinese zoo. Applied Animal Behaviour Science, 96, 93–102.
Davis, C., Crowell, D., & Chun, B. (1965). Monophasic heart rate accelerations in human infants to peripheral stimulation., 478.
Delaney, J. P. A., & Brodie, D. A. (2000). Effects of short-term psychological stress on the time and frequency domains of heart-rate variability. Perceptual and Motor Skills, 2000(91), 515–524.
Derakhchan, K., Chui, R. W., Stevens, D., Gu, W., & Vargas, H. M. (2014). Detection of QTc interval prolongation using jacket telemetry in conscious non-human primates: Comparison with implanted telemetry. British Journal of Pharmacology, 171, 509–522.
Dezecache, G., Zuberbühler, K., Davila-Ross, M., & Dahl, C. D. (2017). Skin temperature changes in wild chimpanzees upon hearing vocalizations of conspecifics. Royal Society Open Science, 4, 160816.
Dobson, A. J., & Barnett, A. G. (2008). An introduction to generalized linear models. CRC Press.
Elsner, B., Pauen, S., & Jeschonek, S. (2006). Physiological and behavioral parameters of infants’ categorization: Changes in heart rate and duration of examining across trials. Developmental Science, 9, 551–556.
Erickson, H. H., & Olsen, S. C. (1985). Electrocardiogram, heart rate, and blood pressure in the chimpanzee. The Journal of Zoo Animal Medicine, 16, 89–97.
Fàbregas, M. C., Guillén-Salazar, F., & Garcés-Narro, C. (2012). Do naturalistic enclosures provide suitable environments for zoo animals? Zoo Biology, 31, 362–373.
Forstmeier, W., & Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner's curse. Behavioral Ecology and Sociobiology, 65, 47–55.
Froesel, M. G. (2020). Automated video-based heart rate tracking for the anesthetized and behaving monkey. Scientific Reports, 1–11.
Graham, F. K., & Clifton, R. K. (1966). Heart-rate change as a component of the orienting response. Psychological Bulletin, 65, 305.
Graham, F. K., & Jackson, J. C. (1970). Arousal systems and infant heart rate responses. Advances in Child Development and Behavior, 5, 59–117.
Graham, F., Anthony, B., & Zeigler, B. (1983). The orienting response and developmental processes. 371–430.
Hajar, R. (2018). The pulse from ancient to modern medicine: Part 3. Heart Views: The Official Journal of the Gulf Heart Association, 19(3), 117.
Hall, M., Vasko, R., Buysse, D., Ombao, H., Chen, Q., Cashmere, J. D., Kupfer, D., & Thayer, J. F. (2004). Acute stress affects heart rate variability during sleep. Psychosomatic Medicine, 66, 56–62.
Hilz, M. J., Stadler, P., Gryc, T., Nath, J., Habib-Romstoeck, L., Stemper, B., & Koehn, J. (2014). Music induces different cardiac autonomic arousal effects in young and older persons. Autonomic Neuroscience, 183, 83–93.
Horschler, D. J., Santos, L. R., & MacLean, E. L. (2019). Do non-human primates really represent others’ ignorance? A test of the awareness relations hypothesis. Cognition, 190, 72–80.
Hyeroba, D. A. (2011). Managing a speared alpha male chimpanzee (pan troglodytes) in Kibale National Park, Uganda. The Veterinary Record, 169(25), 658.
Ing, C., DiMaggio, C., Whitehouse, A., Hegarty, M. K., Brady, J., von Ungern-Sternberg, B. S., & Sun, L. S. (2012). Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics, 130, e476–e485.
Jensen, M. T. (2019). Resting heart rate and relation to disease and longevity: Past, present and future. Scandinavian Journal of Clinical and Laboratory Investigation, 79(1–2), 108–116.
Kano, F., Hirata, S., Deschner, T., Behringer, V., & Call, J. (2016). Nasal temperature drop in response to a playback of conspecific fights in chimpanzees: A thermo-imaging study. Physiology & Behavior, 155, 83–94.
Kearns, K. S., Swenson, B., & Ramsay, E. C. (2000). Oral induction of anesthesia with droperidol and transmucosal carfentonil citrate in chimpanzees (Pan troglodytes). J Zoo Wildlife Med, 31, 185–189.
Kremer, J. J., Foley, C. M., Xiang, Z., Lemke, E., Sarazan, R. D., Osinski, M. A., & Beck, T. W. (2011). Comparison of ECG signals and arrhythmia detection using jacketed external telemetry and implanted telemetry in monkeys. Journal of Pharmacological and Toxicological Methods, 1, e47.
Krumhansl, C. L. (1997). An exploratory study of musical emotions and psychophysiology. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale, 51, 336.
Kutska, D. (2009). Variation in visitor perceptions of a polar bear enclosure based on the presence of natural vs. un-natural enrichment items. Zoo Biology: Published in affiliation with the American Zoo and Aquarium Association, 28, 292–306.
Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26.
Lacey, J. I. (1959). Psychophysiological approaches to the evaluation of psychotherapeutic process and outcome. In E. A. Rubinstein & M. B. Parloff (Eds.), Research in psychotherapy (pp. 160–208). American Psychological Association. https://doi.org/10.1037/10036-010
Lansink, J. M. (1997). Heart rate and behavioral measures of attention in six-, nine-, and twelve-month-old infants during object exploration. Child Development, 68(4), 610–620.
Lowenstine, L. J., McManamon, R., & Terio, K. A. (2016). Comparative pathology of aging great apes: Bonobos, chimpanzees, gorillas, and orangutans. Veterinary Pathology, 53, 250–276.
Maple, T. L., & Finlay, T. W. (1986). Evaluating the environments of captive nonhuman primates. In Primates (pp. 479–488). Springer.
Maple, T. L., & Perdue, B. M. (2013). Zoo animal welfare (vol. 14). Berlin, Germany: Springer.
Maple, T. L., & Stine, W. W. (1982). Environmental variables and great ape husbandry. American Journal of Primatology, 3, 67–76.
McCraty, R. A. M. (1995). The effects of emotions on short-term power spectrum analysis of heart rate variability. The American Journal of Cardiology, 76, 1089–1093.
Melfi, V. A., McCormick, W., & Gibbs, A. (2004). A preliminary assessment of how zoo visitors evaluate animal welfare according to enclosure style and the expression of behavior. Anthrozoös, 17, 98–108.
Ming-Zher Poh, D. J. (2010). Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Optics Express, 10762–10774.
Mireault, G. C., Crockenberg, S. C., Heilman, K., Sparrow, J. E., Cousineau, K., & Rainville, B. (2018). Social, cognitive, and physiological aspects of humour perception from 4 to 8 months: Two longitudinal studies. British Journal of Developmental Psychology, 36, 98–109.
Murphy, H. W., Danforth, M. D., & Clyde, V. L. (2018). The great ape heart project. International Zoo Yearbook, 52, 103–112.
Nomura, S., Yoshimura, K., & Kurosawa, Y. (2013). A pilot study on the effect of music-heart beat feedback system on human heart activity. Journal of Medical Informatics & Technologies, 22, 251–256.
Ogden, J. J., Lindburg, D. G., & MAPLE, T. L. (1993). The effects of ecologically-relevant sounds on zoo visitors. Curator: The Museum Journal, 36, 147–156.
Park, B. J., Tsunetsugu, Y., Kasetani, T., Kagawa, T., & Miyazaki, Y. (2010). The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environmental Health and Preventive Medicine, 15, 18–26.
R Core Team. (2021). R: A language and environment for statistical computing. Retrieved from http://www.R-project.org/
Raper, J., Alvarado, M. C., Murphy, K. L., & Baxter, M. G. (2015). Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology, 123, 1084–1092.
Rasmussen, L. S. (2006). Postoperative cognitive dysfunction: Incidence and prevention. Best Practice & Research Clinical Anaesthesiology, 20, 315–330.
Reynolds, G. D., & Richards, J. E. (2008). Infant heart rate: A developmental psychophysiological perspective.
Richards, J. E. (1992). Development of sustained visual attention in the human infant. Attention and information processing in infants and adults: Perspectives from human and animal research, 30–60.
Sahlin, E., Lindegård, A., Hadzibajramovic, E., Grahn, P., Vega Matuszczyk, J., & Ahlborg, G., Jr. (2016). The influence of the environment on directed attention, blood pressure and heart rate—An experimental study using a relaxation intervention. Landscape Research, 41, 7–25.
Santos, L. R., Barnes, J. L., & Mahajan, N. (2005). Expectations about numerical events in four lemur species (Eulemur fulvus, Eulemur mongoz, Lemur catta and Varecia rubra). Animal Cognition, 8, 253–262.
Schielzeth, H., & Forstmeier, W. (2009). Conclusions beyond support: Overconfident estimates in mixed models. Behavioral Ecology, 20, 416–420.
Shepherdson, D. J. (1998). Tracing the path of environmental enrichment in zoos (pp. 1–12). Environmental enrichment for captive animals.
Shively, C. A. (2015). Social inequalities in health in nonhuman primates. Neurobiology of Stress, 1, 156–163.
Sleigh, J. W., & Henderson, J. D. (1995). Heart-rate-variability and preoperative anxiety. Acta Anaesthesiologica Scandinavica, 39, 1059–1061.
Snyder, D. S. (1984). Peer separation in infant chimpanzees, a pilot study. Primates, 25(1), 78–88.
Sommerfeldt, S. L. (2019). Individual differences in the association between subjective stress and heart rate are related to psychological and physical well-being. Psychological Science, 30(7).
Stekelenburg, J. J. (2002). Pericranial muscular, respiratory, and heart rate components of the orienting response. Psychophysiology, 39(6), 707–722.
Stroud, P. (2007). Defining issues of space in zoos. Journal of Veterinary Behavior, 2, 219–222.
Talpos, J. C., Chelonis, J. J., Li, M., Hanig, J. P., & Paule, M. G. (2019). Early life exposure to extended general anesthesia with isoflurane and nitrous oxide reduces responsivity on a cognitive test battery in the nonhuman primate. Neurotoxicology, 70, 80–90.
Uller, C., Carey, S., Hauser, M., & Xu, F. (1997). Is language needed for constructing sortal concepts? A study with nonhuman primates. Proceedings of the 21st annual Boston University conference on language development, 21, 665–677.
Unakafov, A. M., Möller, S., Kagan, I., Gail, A., Treue, S., & Wolf, F. (2018). Using imaging photoplethysmography for heart rate estimation in non-human primates. PLoS One, 13, e0202581.
Von Borell, E. L.-F. (2007). Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiology & Behavior, 92(3), 293–316.
Walters, J. L., Zhang, X., Talpos, J. C., Fogle, C. M., Li, M., Chelonis, J. J., & Paule, M. G. (2019). Sevoflurane exposure has minimal effect on cognitive function and does not alter microglial activation in adult monkeys. Neurotoxicology, 71, 159–167.
Watts, D. P., & Mitani, J. C. (2001). Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour, 299–327.
Watts, D. P., Muller, M., Amsler, S. J., Mbabazi, G., & Mitani, J. C. (2006). Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. American Journal of Primatology: Official Journal of the American Society of Primatologists, 68, 161–180.
Weissler, A. M., Fineg, J., & Warren, J. V. (1961). The electrocardiogram of the young chimpanzee. Texas Univ Galveston Medical Branch.
Wilson, M. L., & Wrangham, R. W. (2003). Intergroup relations in chimpanzees. Annual Review of Anthropology, 32, 363–392. https://doi.org/10.1146/annurev.anthro.32.061002.120046
Wim Verkruysse, L. O. (2008). Remote plethysmographic imaging using ambient light. Optics Express, 21434–21445.
Winters, S., Dubuc, C., & Higham, J. P. (2015). Perspectives: The looking time experimental paradigm in studies of animal visual perception and cognition. Ethology, 121, 625–640.
Wolf, R. L., & Tymitz, B. L. (1981). Studying visitor perceptions of zoo environments: A naturalistic view. International zoo yearbook, 21, 49–53.
Acknowledgements
This research was partly supported by Draganfly Inc. and the Max Planck Society for the Advancement of Science. We thank Daniel Geissler and all animal keepers at the ape house at the Zoo Leipzig for their support in training the apes to tolerate finger sensor measurements. In particular, we are thankful to Robert Eisenberg for facilitating this collaboration by keeping an open eye on zoo-related research advances. We are also grateful to Steven Kalinke who was vital in setting up the PPG sensor measurement.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding authors
Additional information
Open Practices Statement
None of the data or materials for the experiments reported here is available to other researchers, and none of the experiments was preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Eckert, J., Teague, S. et al. Estimating the cardiac signals of chimpanzees using a digital camera: validation and application of a novel non-invasive method for primate research. Behav Res 56, 2064–2082 (2024). https://doi.org/10.3758/s13428-023-02136-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13428-023-02136-y