Abstract
Speed–accuracy trade-off (SAT) is the tendency for decision speed to covary with decision accuracy. SAT is an inescapable property of aimed movements being present in a wide range of species, from insects to primates. An aspect that remains unsolved is whether SAT extends to plants’ movement. Here, we tested this possibility by examining the swaying in circles of the tips of shoots exhibited by climbing plants (Pisum sativum L.) as they approach to grasp a potential support. In particular, by means of three-dimensional kinematical analysis, we investigated whether climbing plants scale movement velocity as a function of the difficulty to coil a support. Results showed that plants are able to process the properties of the support before contact and, similarly to animal species, strategically modulate movement velocity according to task difficulty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In everyday life, the accuracy of a movement usually depends on its speed. Like the old saying “Haste makes waste”, fast and impulsive movements tend to be more inaccurate than slower and more cautious ones. This behavioral effect termed as speed–accuracy trade-off (SAT) is an inescapable property of choice behavior and has been reported in academic papers dating back more than a century (Beamish, Bhatti, MacKenzie, & Wu, 2006). SAT was first observed by Woodworth (1899), who demonstrated that in a task requiring to move a pencil among target points, the performance resulted in a loss of accuracy when the movement was speeded up.
Fitts refined these early observations formulating a predictive model of human movement (Fitts, 1954; Fitts & Peterson, 1964). According to Fitts (1954), the human sensory-motor “channel” is a limited-capacity system, so that the time to perform an action is proportional to the amount of information required for controlling the movement. This quantity, also known as index of difficulty (ID), depends on the ratio between the size and the distance of the target (Fitts & Peterson, 1964). In particular, according to Fitts’s law (Fitts, 1954; Fitts & Peterson, 1964), when human beings perform movements to targets differing in size (W) and/or positioned at different distances (D), movement time (MT) scales as a logarithmic function of the ratio D/W: MT = a + b × log2(2D/W), with a and b > 0 being respectively the intercept and the slope of the regression line, and log2(2D/W) representing the ID.
A modern explanation of Fitts’s effect is provided by the optimized-submovement model by Meyer and colleagues (Meyer, Abrams, Kornblum, Wright, & Smith, 1988). According to this model, slower movements have a smaller scatter of their end position than faster ones. Undeniably, when the task requires reaching or grasping a smaller or a more distant target, slower movements allow for the acquisition of more information on the target by means of proprioceptive and visual feedback. Using this extra information, any spatial discrepancy between hand and target position is adjusted by implementing corrective adjustments (Meyer et al., 1988; Plamondon & Alimi, 1997).
Fitts’s law has been shown to hold across a wide range of tasks, including movements of the hand (Beggs & Howarth, 1972), the feet (Drury, 1975), hand-held tools (Fitts, 1954), and reach-to-grasp movements (Jakobson & Goodale, 1991). Furthermore, SAT mechanisms are also at work in various animal species (Chittka, Skorupski, & Raine, 2009). For instance, the motor control of many animals, such as house-hunting ants (Franks, Dornhaus, Fitzsimmons, & Stevens, 2003), monkeys (Heitz & Schall, 2012), birds (Ducatez, Audet, & Lefebvre, 2015), and fish (Burns & Rodd, 2008), is influenced by the size and the distance of the target, as predicted by Fitts’s law.
To date, a great absent in the Fitts’s law literature is the “green kingdom.” At first glance, plants seem relatively immobile, stuck to the ground in rigid structures and, unlike animals, unable to escape stressful environments. But, although markedly different from those of animals, movement pervades all aspects of plant behavior (Darwin & Darwin, 1880). As observed by Darwin (1875), the tendrils of climbing plants undergo subtle movements around their axes of elongation. This elliptical movement, known as circumnutation, allows plants to explore their immediate surroundings in search, for instance, of a physical support to enhance light acquisition (Larson, 2000). Also, Darwin (1875; see also Trewavas, 2017) observed that the tendrils tend to assume the shape of whatever surface before they come into contact with. Implicitly this might signify that they “see” the support and plan the movement accordingly. In this view, climbing plants might be able to plan the course of an action ahead of time and program the tendrils’ choreography according to the “to-be-grasped” object.
Support for this contention comes from both theoretical and empirical studies suggesting that plant movement is not a simple product of cause–effect mechanisms but rather seems to be driven by processes that are anticipatory in nature (e.g., Calvo & Friston, 2017; Guerra et al., 2019). For instance, a recent study shows that a climbing plant (Pisum sativum L.) not only is able to perceive a potential support, but it also scales the kinematics of tendrils’ aperture according to its size well ahead they touch the stimulus (Guerra et al., 2019). This has been taken as the demonstration that plants plan the movement purposefully and in ways that are flexible and anticipatory.
Continuing on this analysis, here we ask whether the movement of plants obeys Fitts’s law. We explore the influence of the support’s diameter on the approaching phase of climbing plants—that is, the movement by which plants close the gap in between their tendrils and a support standing in their vicinity. This movement has a crucial importance for plants and influences their long-term chances for survival. Finding a climbable trellis, as fast and as accurately as possible, allows plants to avoid shading by co-occurring taller plants and enhance light acquisition, as well as place climbers beyond ground herbivores (Gianoli, 2015).
With this in mind, one of the empirical predictions stemming from Fitts’s law can be well-suited to model the three-dimensional circumnutation of plants. Precisely, we refer to the evidence that movement time scales as a function of the target’s size: When the distance is constant, thinner targets are reached more slowly than thicker ones (see Murata & Iwase, 2001). We test this prediction in Pisum sativum L. by assessing the change of velocity of the tendrils during their approach-to-grasp a thin or to a thicker support.
Method
Subjects
A total of 10 snow peas (Pisum sativum var. saccharatum cv Carouby de Maussane) were used in the present experiment. Pea is an annual plant from the Fabaceae family growing filamentous organs, called tendrils. Tendrils have the ability to twine around other structures and allow pea plant to climb a support in order to wend its way to sunlight and other numerous ecological niches (Wang, Li, Cho, & Ahn, 2018). We did not perform a power analysis to determine the sample size, because SAT mechanisms have never been investigated in plants. Thus, running a power analysis is not recommended because it may lead to arbitrary decisions concerning sample size. Plants were randomly assigned to each experimental condition.
Stimulus
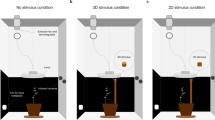
The stimulus was a wooden support, 60-cm high, and either 3 cm (thick stimulus) or 1.2 cm (thin stimulus) in diameter, positioned at a distance of 12 cm in front of the first unifoliate leaf for each plant (see Fig. 1a).
Apparatus
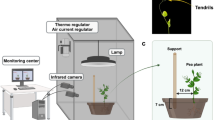
The experimental apparatus is described in Fig. 1a. Pea seeds were sowed in cylindrical pots (⌀ = 20 cm, height = 20 cm) containing agricultural soil. We sowed one seed per pot, at a depth of 2.5 cm, by placing the seed at a distance of 6 cm from pot center. Pots were then enclosed in growth chambers (Cultibox SG combi 80 × 80 × 10 cm) for germination and growth in controlled environment conditions. Chamber air temperature was set at 26 °C by an extractor fan equipped with a thermo-regulator (TT125; 125-mm diameter; max 280 MC/H vents) and an input-ventilation fan (Blauberg Tubo 100–102 m3/h). The combination of the two fans allowed for a steady air circulation into the growth chamber with an air mean residence time of 60 seconds. The disposition of the fan was such that air circulation did not affect the plants’ natural movements. Plants were grown with a 11.25-hour photoperiod (5.45 a.m. to 5 p.m.) under a cool white LED lamp (V-TAC innovative LED lighting, VT-911-100W, Des Moines, IA, USA) that was exactly centered at 50 cm above each seedling. Photosynthetic photon flux density at 50 cm under the lamp in correspondence of the seedling was umolPh m-2 s-1 (quantum sensor LI-190R, Lincoln, Nebraska USA). Pots were watered with tap water as needed three times a week. Experimental treatments were applied to single plants while individually growing in one growing chamber. Treatments were replicated five times by randomly assigning treatments to the four growing chambers.
Video recording and data analysis
The recording of plants’ movement was performed using a pair of RGB-infrared cameras (i.e., IP 2.1 MPX outdoor varifocal IR 1080P) placed inside each growth chamber, 110 cm off the ground and spaced at a distance of 45 cm to record stereo images of the plant. The cameras were connected through ethernet cables to a 10-port wireless router (i.e., D-Link DSR-250n) via wi-fi to a PC on which the frames acquisition and saving process was controlled by means of CamRecorder software (Ab.Acus S.r.l., Milan, Italy). Black felt velvet was fixed to all sectors of the growth chambers walls and the wooden stimuli were darkened with charcoal in order to maximize the visibility of the tendrils by the cameras. The intrinsic, extrinsic, and lens distortion parameters of each camera were estimated using MATLAB Camera Calibrator app. Twenty pictures of a chessboard (10 columns and 7 rows of squares 18 × 18 mm) taken from multiple angles and distances, in natural nondirect light conditions were used for the single-camera parameters extraction process. The same chessboard was placed in the middle of the growth chamber and used for the calibration of each camera. In particular, a picture was taken by the two cameras, to extract the stereo calibration parameters. In the experimental protocol, each frame was acquired every 3 minutes (frequency 0.0056 Hz) synchronously from each camera of the growth chamber. To track the position of the markers and reconstruct their 3-D trajectory, we used an ad hoc software (Ab.Acus S.r.l., Milan, Italy) developed in MATLAB. The initial frame was defined as the frame at which the tendrils of the coiled leaf were visible from the apex. The end of the plant movement was defined as the time at which the tendrils started to coil the support. The end of plant movement was independently verified by a researcher blind to condition. The anatomical landmarks of interest—namely, the tips of the tendrils—were considered as markers and tracked off-line (see Fig. 1b). Two markers were also positioned on the top and the bottom of the support and served as reference points. Tracking procedures were performed at first automatically throughout the time course of the movement sequence using Kanade–Lucas–Tomasi (KLT) algorithm on the frames acquired by each camera, after distortion removal. The tracking was manually verified by the experimenter, who checked the position of the markers frame by frame. The 3-D trajectory of each tracked marker was computed by triangulating the 2-D trajectories obtained from the two cameras. The dependent measures were calculated on the bases of the anatomical landmarks of interest for each plant (see Fig. 1b). We considered the movement time (the time between the initial frame and the end of plant movement), the maximum tendrils velocity (the maximum velocity reached by tendrils during the movement time), and the average tendrils velocity (the mean velocity of tendrils during the movement time).
Statistical analysis
Statistical analyses were performed using the Bayesian approach, which provides an accurate parameter estimation. On the contrary, the frequentist approach runs a strong risk of false negatives for small samples (Vadillo, Konstantinidis, & Shanks, 2016). In Bayesian estimation the aim is to allocate credibility to a distribution of possible parameter values (posterior distribution) consistent with the observed data, by generating a large number of samples by using a Markov chain Monte Carlo method (MCMC). Next, 95% high-density intervals (95% HDI) are calculated to define which points of the distribution are most credible, and which cover most of the distribution (for a review, see Kruschke, 2013). In the present study, we performed a Bayesian t test to compare the mean of the “thin” (μthin) and the “thick” (μthick) stimulus conditions. For this purpose, using the BEST (Bayesian ESTimation supersedes the t test) model implemented by Kruschke (2013), we calculated the difference (β) between the mean of the thin (μthin) and the thick (μthick) stimulus conditions, and compared the credibility of β > 0 with β < 0. All statistical analyses were performed using the computing environment R (R Core Team, 2014), and the packages BEST (Kruschke & Meredith, 2018) under the default setting.
Results
Qualitative description of movement
As shown in Fig. 2, the analysis of the spatial trajectories revealed that all the considered landmarks showed a growing pattern characterized by circumnutation (Darwin, 1875; see Fig. 2)—that is, an elliptical movement performed by plants during growth. At a certain stage of circumnutation, the tendrils veered towards the stimulus and stopped at the time the plant grasped the support.
Kinematical results
Movement time
The mean difference of movement time between the thin (μthin = 2,093 min ± 445) and the thick (μthick = 3,082 min ± 1,650) Stimulus condition was β = −989 mm/min, with a 95% uncertainty interval ranging from −4,230 to 2,030 min (see Fig. 3a). The probability of β < 0 (i.e., the probability that the movement time was shorter for the thin than for the thick stimulus condition) was 79.3%, whereas the probability of β > 0 (i.e., the probability that the movement time was shorter for the thick stimulus condition) was 20.7%.
Maximum tendrils velocity
The mean difference of the maximum tendril’s velocity between the thin (μthin = 16.32 mm/min ± 3.70) and the thick (μthick = 6.53 mm/min ± 0.91) Stimulus condition was β = 9.79 mm/min, with a 95% uncertainty interval ranging from 2.39 to 17.50 mm/min (see Fig. 3b). The probability of β > 0 (i.e., the probability that the maximum tendrils velocity was larger for the thin than for the thick stimulus condition) was 99.3%, whereas the probability of β < 0 (i.e., the probability that the maximum tendrils velocity was larger for the thick stimulus condition) was 0.7%
Average tendrils velocity
The mean difference of the average tendrils velocity between the thin (μthin = 1.88 mm/min ± 0.27) and the thick (μthick = 1.09 mm/min ± 0.16) stimulus condition was β = 0.79 mm/min, with a 95% uncertainty interval ranging from 0.14 to 1.41 mm/min (see Fig. 3c). The probability of β > 0 (i.e., the probability that the average tendrils velocity was larger for the thin than for the thick stimulus condition) was 99.2%, whereas the probability of β < 0 (i.e., the probability that the average tendrils velocity was larger for the thick stimulus condition) was 0.8.
Discussion
In the present study, we investigated, for the first time, whether the movement velocity of pea tendrils scales as a function of support’s diameter, as predicted by Fitts’s law. The results showed that peas’ scale movement velocity during circumnutation with respect to the size of the support. The average and the maximum tendrils velocity were faster when plants had to reach and grasp a thinner than a thicker support. The analysis of movement time confirms this evidence, showing that movement time was shorter for the thinner than for the thicker stimulus (β < 0) with a probability of 79.3%. This evidence suggests that plants are able to process the properties of the support and are endowed with a form of perception underwriting a goal-directed and anticipatory behavior (Guerra et al., 2019). However, in contrast with previous human and animal literature (e.g., Beggs & Howarth, 1972; Fitts, 1954; Heitz & Schall, 2012), our results indicate an opposite pattern of what Fitts’s law predicts. Remember that according to Fitts’s law, the velocity of the movement is inversely proportional to ID (2D/W). In other words, our results seem to suggest that plants exhibit more difficulty grasping a thicker than a thinner support. These findings are line with previous reports showing a lower success rate of attachment for thick supports (Peñalosa, 1982), and a preference for plants to climb supports with a smaller diameter (Darwin, 1875; Putz, 1984; Putz & Holbrook, 1992). Furthermore, by using the curvature of tendrils during the twining phase, Goriely and Neukirch (2006) demonstrate that for thinner supports, the contact angle (i.e.t, the angle between the tip of the tendril and the tangent of the support) is a near-zero value. Instead, with thicker supports, the contact angle tends to increase as tendrils must curl into the support’s surface to maintain an efficient grip. When the support is too thick, the contact angle increases to an extent that the tendril curls back on itself, losing grip. Interestingly, field studies in rainforests showed that the presence of climbing plants tends to decrease in areas in which there is a prevalence of thicker supports (Carrasco-Urra & Gianoli, 2009).
A possible explanation for this phenomenon may reside in the fact that, for plants, reaching to grasp thick supports is a more energy consuming process than grasping for thinner ones. Indeed, the grasping of a thick support implies that plants have to increase the tendrils length in order to efficiently coil the support (Rowe, Isnard, Gallenmüller, & Speck, 2006), and to strengthen the tensional forces to resist gravity (Gianoli, 2015). Since these processes are characterized by a high adenosine triphosphate (ATP) consumption, coiling thicker supports results in more energy expenditure (Putz & Holbrook, 1992). Coherently, the reduction of movement velocity during the approaching maneuver may allow climbing plants to preserve energy for the coiling phase so as to reduce the risk of errors and assure a firm attachment to the support. In this sense, the SAT of plants may be mediated by the consumption of energy.
The reduction of movement velocity may also serve to lengthen the time window within which tendrils establish contact points with the support. Previous literature has shown that climbing plants strategically modify contact points when twining around supports of different diameters (Putz & Holbrook, 1992). Therefore, the extra time needed to reach a thicker support may allow climbing plants to correct tendrils trajectories and more accurately select contact points in order to more firmly to twine around the support. This interpretation is consistent with the most prominent theory of the SAT—the optimized-submovement model by Meyer et al. (1988). Indeed, when plants have no other choice but to climb a thick support, it might be necessary to slow down their movement so as to accumulate more evidence about the support’s physical characteristics and implement corrective adjustments to reduce the scatter of the tendrils’ end position.
The mechanisms by which plants could perceive the differences between support sizes remains to be explained. Based on the evidence that plants have at their disposal a great variety of sensory modalities (Karban, 2015), we hypothesize three possible situations. First, plants may use echolocation to acquire information about the support. Recent reports showed that plants emit sonic clicks and capture the returning echoes to get information about their surroundings (Gagliano, Renton, Duvdevani, Timmins, & Mancuso, 2012). This bio sonar may provide information about the thickness of the support to the plants, which will act accordingly. Second, several studies have suggested that the leaf’s upper and subepidermis comprise cells acting as ocelli, eye-like structures allowing plants to gather visual information about their environment (Baluška & Mancuso, 2016). Support for this contention comes from studies on Boquilla trifoliolata, a climbing wood vine that modifies the appearance of its leaves according to the host plant, perfectly mimicking the colors, shapes, sizes, orientations, and petiole lengths of the leaves. Crucially, the plant leaf mimicry occurs even without a direct contact between the vine of Boquilla trifoliolata and mimicked host trees, which supports the idea that plants are capable not only of sensing but also of decoding visual inputs (Gianoli & Carrasco-Urra, 2014). Thus, climbing plants may benefit from a vision system that is able to process the proprieties of the support. Lastly, plants may acquire information about the support using chemoreception of volatiles. It is well known that plants release airborne chemicals that can convey ecologically relevant information about the stimuli they interact with (Karban, 2015; Runyon, Mescher, & De Moraes, 2006). Based on our measurements, we cannot single out the specific mechanism involved in the size coding of plants, and therefore further research is needed to clarify this aspect.
Another aspect stemming from this study is its empirical support to the construction of a solid theory for plant cognition (Calvo, 2007; Parise, Gagliano, & Souza, 2020; Segundo-Ortin & Calvo, 2019). For instance, plants have been excluded from the wider cognitive domain as it was thought that they lacked the sensorimotor organization that characterizes animals (Calvo & Keijzer, 2009). Because plants do not have freedom of movement within their environment, it was concluded that they lack the precondition for cognitively guided behavior. But it may not be necessary to link cognition with free movement within an environment. As we demonstrate, while plants are stuck in the ground, they are nevertheless able to integrate internal and external signals, displaying complex behaviors, such as the one described here. And some plants’ movements achieve many of the same functional ends as those of animals (Guerra et al., 2019).
In conclusion, plants seem to benefit from strategic SAT mechanisms, until recently considered the preserve of brained organisms. Further research should consider a wider array of sizes and distances to corroborate the reliability of the present results. Nevertheless, the result of this study speaks against the idea that the movement of plants is only driven by cause–effect mechanisms and hard-wired reflexes. Rather, they point to the ability of planning and executing movement in a flexible and anticipatory manner.
Open practices statements
This experiment was not preregistered. All relevant data and R scripts are available (https://osf.io/vg5u3/).
References
Baluška, F., & Mancuso, S. (2016). Vision in plants via plant-specific ocelli? Trends in Plant Science, 21(9), 727–730. doi:https://doi.org/10.1016/j.tplants.2016.07.008
Beamish, D., Bhatti, S. A., MacKenzie, I. S., & Wu, J. (2006). Fifty years later: A neurodynamic explanation of Fitts’s law. Journal of the Royal Society Interface, 3(10), 649–654. doi:https://doi.org/10.1098/rsif.2006.0123
Beggs, W. D. A., & Howarth, C. I. (1972). The movement of the hand towards a target. Quarterly Journal of Experimental Psychology, 24(4), 448–453. doi:https://doi.org/10.1080/14640747208400304
Burns, J. G., & Rodd, F. H. (2008). Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Animal Behaviour, 76(3), 911–922. doi:https://doi.org/10.1016/j.anbehav.2008.02.017
Calvo, P. (2007). The quest for cognition in plant neurobiology. Plant Signaling & Behavior, 2(4), 208–211. doi:https://doi.org/10.4161/psb.2.4.4470
Calvo, P., & Friston, K. (2017). Predicting green: Really radical (plant) predictive processing. Journal of the Royal Society Interface, 14(131), 20170096. doi:https://doi.org/10.1098/rsif.2017.0096
Calvo, P., & Keijzer, F. A. (2009). Cognition in plants. In F. Baluška (Ed.), Plant–environment interactions: Behavioral perspective (pp. 247–266). Berlin, Germany: Springer-Verlag.
Carrasco-Urra, F., & Gianoli, E. (2009). Abundance of climbing plants in a southern temperate rain forest: Host tree characteristics or light availability? Journal of Vegetation Science, 20(6), 1155–1162. doi:https://doi.org/10.1111/j.1654-1103.2009.01115.x
Chittka, L., Skorupski, P., & Raine, N. E. (2009). Speed–accuracy trade-offs in animal decision making. Trends in Ecology & Evolution, 24(7), 400–407. doi:https://doi.org/10.1016/j.tree.2009.02.010
R Core Team. (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org
Darwin, C. (1875). The movements and habits of climbing plants. London, England: John Murray.
Darwin, C., & Darwin, F. (1880). The power of movement in plants. London, England: John Murray.
Drury, C. G. (1975). Application of Fitts’s law to foot-pedal design. Human Factors: The Journal of the Human Factors and Ergonomics Society, 17(4), 368–373. doi:https://doi.org/10.1177/001872087501700408
Ducatez, S., Audet, J. N., & Lefebvre, L. (2015). Problem-solving and learning in Carib grackles: Individuals show a consistent speed–accuracy trade-off. Animal Cognition, 18(2), 485–496. doi:https://doi.org/10.1007/s10071-014-0817-1
Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47(6), 381–391. doi:https://doi.org/10.1037/h0055392
Fitts, P. M., & Peterson, J. R. (1964). Information capacity of discrete motor responses. Journal of Experimental Psychology, 67(2), 103–112. doi:https://doi.org/10.1037/h0045689
Franks, N. R., Dornhaus, A., Fitzsimmons, J. P., & Stevens, M. (2003). Speed versus accuracy in collective decision making. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1532), 2457–2463. doi:https://doi.org/10.1098/rspb.2003.2527
Gagliano, M., Renton, M., Duvdevani, N., Timmins, M., & Mancuso, S. (2012). Out of sight but not out of mind: Alternative means of communication in plants. PLOS ONE, 7(5). doi:https://doi.org/10.1371/journal.pone.0037382
Gianoli, E. (2015). The behavioural ecology of climbing plants. AoB PLANTS, 7, plv013. doi:https://doi.org/10.1093/aobpla/plv013
Gianoli, E., & Carrasco-Urra, F. (2014). Leaf mimicry in a climbing plant protects against herbivory. Current Biology, 24(9), 984–987. doi:https://doi.org/10.1016/j.cub.2014.03.010
Goriely, A., & Neukirch, S. (2006). Mechanics of climbing and attachment in twining plants. Physical Review Letters, 97(18), 184302. doi:https://doi.org/10.1103/PhysRevLett.97.184302
Guerra, S., Peressotti, A., Peressotti, F., Bulgheroni, M., Baccinelli, W., D’Amico, E. ,… Castiello, U. (2019). Flexible control of movement in plants. Scientific Reports, 9(1), 1–9.
Heitz, R. P., & Schall, J. D. (2012). Neural mechanisms of speed–accuracy trade-off. Neuron, 76(3), 616–628. doi:https://doi.org/10.1016/j.neuron.2012.08.030
Jakobson, L. S., & Goodale, M. A. (1991). Factors affecting higher-order movement planning: A kinematic analysis of human prehension. Experimental Brain Research, 86(1), 199–208. doi:https://doi.org/10.1007/bf00231054
Karban, R. (2015). Plant sensing and communication. Chicago, IL: University of Chicago Press.
Kruschke, J. K. (2013). Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General, 142(2), 573–603. doi:https://doi.org/10.1037/a0029146
Kruschke, J. K., & Meredith, M. (2018). BEST: Bayesian estimation supersedes the t-test (R Package Version 0.5.0) [Computer software]. Retrieved from http://CRAN.R-project.org/package=BEST
Larson, K. C. (2000). Circumnutation behavior of an exotic honeysuckle vine and its native congener: Influence on clonal mobility. American Journal of Botany, 87(4), 533–538. doi:https://doi.org/10.2307/2656597
Meyer, D. E., Abrams, R. A., Kornblum, S., Wright, C. E., & Smith, J. E. K. (1988). Optimality in human motor performance: Ideal control of rapid aimed movements. Psychological Review, 95(3), 340–370. doi:https://doi.org/10.1037/0033-295x.95.3.340
Murata, A., & Iwase, H. (2001). Extending Fitts’s law to a three-dimensional pointing task. Human Movement Science, 20(6), 791–805. doi:https://doi.org/10.1016/s0167-9457(01)00058-6
Parise, A. G., Gagliano, M., & Souza, G. M. (2020). Extended cognition in plants: Is it possible? Plant Signaling & Behavior, 1710661. doi:https://doi.org/10.1080/15592324.2019.1710661
Peñalosa, J. (1982). Morphological specialization and attachment success in two twining lianas. American Journal of Botany, 69(6), 1043–1045. doi:https://doi.org/10.1002/j.1537-2197.1982.tb13348.x
Plamondon, R., & Alimi, A. M. (1997). Speed/accuracy trade-offs in target-directed movements. Behavioral and Brain Sciences, 20(2), 279–303. doi:https://doi.org/10.1017/s0140525x97001441
Putz, F., & Holbrook, N. (1992). Biomechanical studies of vines. In F. Putz & H. Mooney (Eds.), The biology of vines (pp. 73–98). Cambridge, England: Cambridge University Press. doi:https://doi.org/10.1017/CBO9780511897658.005
Putz, F. E. (1984). The natural history of lianas on Barro Colorado Island, Panama. Ecology, 65(6), 1713–1724. doi:https://doi.org/10.2307/1937767
Rowe, N. P., Isnard, S., Gallenmüller, F., & Speck, T. (2006). Diversity of mechanical architectures in climbing plants: An ecological perspective. In A. Herrel, T. Speck, & N. P. Rowe (Eds.), Ecology and biomechanics: A mechanical approach to the ecology of animals and plants (pp. 35–59). Boca Raton, FL, CRC Press.
Runyon, J. B., Mescher, M. C., & De Moraes, C. M. (2006). Volatile chemical cues guide host location and host selection by parasitic plants. Science, 313(5795), 1964–1967. doi:https://doi.org/10.1126/science.1131371
Segundo-Ortin, M., & Calvo, P. (2019). Are plants cognitive? A reply to Adams. Studies in History and Philosophy of Science Part A, 73, 64–71. doi:https://doi.org/10.1016/j.shpsa.2018.12.001
Vadillo, M. A., Konstantinidis, E., & Shanks, D. R. (2016). Underpowered samples, false negatives, and unconscious learning. Psychonomic Bulletin & Review, 23(1), 87–102. doi:https://doi.org/10.3758/s13423-015-0892-6
Wang, W., Li, C., Cho, M., & Ahn, S. H. (2018). Soft tendril-inspired grippers: Shape morphing of programmable polymer–paper bilayer composites. ACS Applied Materials & Interfaces, 10(12), 10419–10427. doi:https://doi.org/10.1021/acsami.7b18079
Woodworth, R. S. (1899). Accuracy of voluntary movement. The Psychological Review: Monograph Supplements, 3(3), 1–114. doi:https://doi.org/10.1037/h0092992
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ceccarini, F., Guerra, S., Peressotti, A. et al. Speed–accuracy trade-off in plants. Psychon Bull Rev 27, 966–973 (2020). https://doi.org/10.3758/s13423-020-01753-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-020-01753-4