Abstract
Eye gaze conveys rich information concerning the states of mind of others, playing a critical role in social interactions, signaling internal states, and guiding others’ attention. On the basis of its social significance, some researchers have proposed that eye gaze may represent a unique attentional stimulus. However, contrary to this notion, the majority of the literature has shown indistinguishable attentional effects when eye gaze and arrows have been used as cues. Taking a different approach, in this study we aimed at finding qualitative attentional differences between gazes and arrows when they were used as targets instead of as cues. We used a spatial Stroop task, in which participants were required to identify the direction of eyes or arrows presented to the left or the right of a fixation point. The results showed that the two types of stimuli led to opposite spatial interference effects, with arrows producing faster reaction times when the stimulus direction was congruent with the stimulus position (a typical spatial Stroop effect), and eye gaze producing faster reaction times when it was incongruent (a “reversed” spatial Stroop effect). This reversed Stroop is interpreted as an eye-contact effect, therefore revealing the unique nature of eyes as special social-attention stimuli.
Similar content being viewed by others
Others’ gazes constitute an essential medium through which humans can communicate socially relevant information, such as their focus of interest, private thoughts, and intentions (e.g., Baron-Cohen, Wheelwright, & Jolliffe, 1997). Visual attention is also deeply modulated by eye gaze (e.g., Frischen, Bayliss, & Tipper, 2007) and our ability to attend the same object as an observed individual serves as a foundation for more sophisticated social skills such as a theory of mind, language acquisition and cultural learning (Tomasello, 1995). Indeed, averted-gaze stimuli can produce both covert (e.g., Driver et al., 1999) and overt (e.g., Kuhn & Benson, 2007) attentional orienting, whose magnitude is modulated by the social salience of these stimuli (e.g., Dalmaso, Galfano, & Castelli, 2015).
Direct gaze can also impact on several cognitive and attentional mechanisms (i.e., the “eye contact effect”; Senju & Johnson, 2009), presumably due to the salience associated with such an indicator of being attended to. For examples, direct gaze can improve face processing (Macrae, Hood, Milne, Rowe, & Mason, 2002), increase arousal in the recipient of gaze (e.g., Conty et al., 2010), and signal approach (Hietanen, Leppänen, Peltola, Linnaaho, & Ruuhiala, 2008).
On the basis of these findings, it has been argued that attention to eye gaze may represent a unique attentional process and reflect the operation of a specialized cognitive mechanism. Thus, in the last years, to evaluate the uniqueness of the eye gaze, many studies have tried to dissociate gaze attentional mechanisms from the attentional mechanisms engaged by symbolic directional stimuli such as arrows. However, although a variety of research strategies has been used, no general agreement has yet been achieved, and some authors (e.g., Santiesteban, Catmur, Hopkins, Bird, & Heyes, 2014), observing very similar effects for gazes and arrows, have proposed that gaze attentional effects are at least partially driven by a domain-general attentional processing.
Thus, an initial comparison between gaze and arrow cues has shown that eye-gaze cues are more resistant to voluntary control (Friesen, Ristic, & Kingstone, 2004). In particular, Friesen et al. used a so-called counterpredictive cueing paradigm (the target was more likely to appear in the location opposite the one indicated by the cue) and showed that better performance at the indicated location was only observed when eye gaze was used as cue, but not when the indicated location was cued by an arrow. In contrast, when counterpredictive cueing was tested with arrows, participants’ attention did not shift to the cued locations. However, in a more recent study using the same counterpredictive paradigm, Tipples (2008) found that both eye and arrow cues produce similar reflexive shifts of attention.
Similarly, different overt orienting (involving eye movements) of attention for central gaze cues and arrow cues has been shown in one study (Ricciardelli, Bricolo, Aglioti, & Chelazzi, 2002), whereas subtle or no differences have been shown in others (Kuhn & Benson, 2007; Kuhn & Kingstone, 2009). Taken together, these results seem to suggest that gaze produces attentional effects that do not differ substantially from those produced by arrow cues.
Recently, however, using a more powerful “qualitative” approach, several authors have found clear dissociations between gaze and arrow attentional effects. The logic here is that the attentional differences between gaze and arrow cues might be regarding the nature rather than the size of the attentional modulation induced by each cue type. For example, by using a variant of the double-rectangle task, Marotta, Lupiáñez, Martella, and Casagrande (2012) showed that attention spread to the entire cued object when arrows were used as cues, whereas it was selectively directed to the specific location or part of the object looked at, when gaze cues were used. Moreover, Bayliss, Paul, Cannon, and Tipper (2006) found that objects that are looked at by other people are more likable than those that do not receive much attention from others. This affective preference for cued objects was not found when arrows cues were used. Finally, combining a traditional gaze-cueing paradigm with a visual memory task, Dodd, Weiss, McDonnell, Sarwal, and Kingstone (2012) and Gregory and Jackson (2017) have shown that gaze cues but not arrow cues improved memory accuracy for cued information.
Although the dissociations between gaze and arrow cues described above are consistent with the view that eye gaze represents a unique and special attention cue, a weakness in the overall pattern of findings is noteworthy: namely, the absence of an effect of a particular type of cue could reflect a lack of sensitivity of the experimental procedure rather than a real difference between gaze and arrow cues. Ideally, powerful dissociation would involve a single task in which participants’ performance would be modulated in opposite ways by gaze and arrow cues.
Seeking this type of qualitative double dissociation, the present study aimed at evaluating how gaze and arrows leads to spatial interference in response selection. One of the main tools to study the influence of irrelevant spatial information on performance is the spatial Stroop task (see Lu & Proctor, 1995, for a review). In its most-used variant (Funes, Lupiáñez, & Milliken, 2007; Pires, Leitão, Guerrini, & Simões, 2017), a directional arrow is randomly presented to the left or right side of a fixation point and participants are required to discriminate the direction of the arrow while ignoring its location. The results have generally shown faster and more accurate responses to congruent stimuli (e.g., a right-pointing arrow presented on the right) than to incongruent ones (e.g., a left-pointing arrow presented on the right). However, in a recent study using a variant of the spatial Stroop paradigm in which eye gazes were used as the stimuli, Cañadas and Lupiañez (2012) found a reversed congruency effect, with faster reaction times (RTs) to incongruent stimuli (e.g., a face looking to the left, presented on the right). Although these findings suggest the existence of an important dissociation between gaze and arrow stimuli, it constitutes a dissociation between different task contexts and experimental settings. Task differences make it difficult to directly compare the effects produced by the two types of stimuli. For this reason, in the present study we sought to directly compare the influence on performance of gaze and arrow stimuli in a context of the spatial Stroop task.
Consistent with the majority of the findings obtained in the literature (Funes et al., 2007; Pires et al., 2017), we expected arrow stimuli to produce a typical spatial Stroop effect, with faster RTs for congruent than for incongruent stimuli. If gaze stimuli were also to produce spatial Stroop interference, this result would favor the domain-general attentional process view. In contrast, if, consistent with our predictions, gaze stimuli were to produce an effect that was opposite from the spatial Stroop effect—that is, faster RTs for incongruent stimuli—this result would be strongly consistent with the view that eye gaze represents a unique and special attentional stimulus.
Method
Participants
Informed consent was obtained form 36 students (27 women, nine men), with a mean age of 20.64 years, from the University of Granada. They received partial course credit for participating. All had self-reported normal or corrected-to-normal vision and were naïve as to the purpose of the experiment. We estimated the required sample size assuming a significance level of .05 and a power of .8, taking as a reference the effect size obtained in Jones (2015, Exp. 1).
Apparatus and stimuli
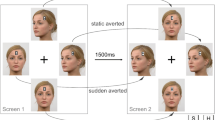
The stimulus presentation, timing, and data collection were controlled by a program written using E-Prime 2.0, run on a standard Pentium 4 PC. Stimuli were presented on a 17-in. widescreen monitor with a 1,024 × 768 pixel resolution. They consisted of a 1 × 4 cm display of two black arrows or of two full-color cropped eyes on a gray rectangle (see Fig. 1). The cropped eyes were obtained by manipulating an original face (taken from the MacBrain Face Stimulus Set; www.macbrain.org/faces/index.htm)Footnote 1 with Adobe Photoshop CS.
Procedure
Participants were seated approximately 60 cm from the computer screen in a faintly lit room to perform the experimental task. They were required to perform a discrimination task in which they had to respond as fast and accurately as possible to the direction (left or right) indicated by the eye gaze or arrows. The experiment was composed of two halves (one for each target type), each one composed of 15 practice trials followed by two experimental blocks of 64 trials each. Target direction and target location were randomly selected within each block of trials. The target types (gaze/arrow) were separated in different halves of the experiment, with the order counterbalanced across participants.
Each trial (see Fig. 1) began with a white fixation cross presented in the center of a black screen for 1 s. Participants were instructed to fixate the cross. Then a pair of eyes or arrows looking/pointing to the right or to the left was presented to either the left or the right of the fixation cross for 2 s. The distance from the center of the lateral stimulus to the central fixation cross was 5 cm. Participants were instructed to press the “Z” key in response to targets indicating the left and the “M” key in response to targets indicating the right, independent of the target’s location. Feedback for incorrect key presses was provided to participants, in the form of a 220-Hz tone presented for 1,500 ms.
Importantly, this design produced trials that were congruent (e.g., a right-indicating target presented on the right) or incongruent (e.g., a left-indicating target presented on the right).
Design
The experiment had a two-factor repeated measures design, with 64 observations per experimental condition. Target type had two levels: gaze and arrow. Trial type had two levels: congruent and incongruent trials. Partial analyses of variance (ANOVAs) were conducted to analyze the interactions. For each participant, the mean RTs and accuracy (as mean percent errors) were calculated for each experimental condition.
Results
As in Cañadas and Lupiáñez (2012), RTs faster than 200 ms (0.13% of trials) or slower than 1,300 ms (0.56%), as well as trials with an incorrect responses (6.45%), were excluded from the RT analysis. The data of one participant were removed prior to analysis because the accuracy rate in two of the four blocks was 50%, and inspection of the responses revealed that this participant had been tapping an incorrect key. Table 1 shows the means (± SDs) of the RTs and percentages of errors for each experimental condition.
Reaction times
The ANOVA performed on mean RTs showed a main effect of target type, F(1, 34) = 133.52, p ˂ .001, ηp2 = .80, with faster RTs for the arrow targets than for the gaze targets (508 vs. 588 ms). The main effect of trial type was not significant, F(1, 35) < 1, p = .553, ηp2 = .01. Importantly, the critical Target Type × Trial Type interaction was significant, F(1, 34) = 39.76, p < .001, ηp2 = .54 (Fig. 2). Partial ANOVAs on each target type showed that RTs were significantly longer on incongruent trials (519 ms) than on congruent trials (497 ms) when arrows were used as the targets, F(1, 34) = 17.59, p < .001, ηp2 = .34; in contrast, RTs were significantly faster on incongruent trials (580 ms) than on congruent trials (597 ms) when eye gaze was used as the target, F(1, 34) = 8.26, p = .007, ηp2 = .20.
Mean reaction times for each target type and trial type condition. Error bars represent standard errors of the means, with between-participants variance removed using Cousineau’s (2005) method.
Errors
Neither the main effect of target type, F(1, 34) < 1, p = .885, ηp2 < .01, nor the main effect of trial type, F(1, 34) = 1.09, p = .304, ηp2 = .03, was significant. However, of relevance for the present study, the Target Type × Trial Type interaction was significant, F(1, 34) = 6.61, p = .015, ηp2 = 0.71. It is important to note that the error data were in the same direction as the RTs: In the arrow target condition, participants made more errors on incongruent than on congruent trials, F(1, 34) = 9.20, p = .005, ηp2 = .21, whereas in the gaze target condition they made more errors on congruent than on incongruent trials, although this difference was not significant, F(1, 34) < 1, p = .427, ηp2 = .02.
Discussion
In the present study, we observed that the eye-gaze and arrow stimuli led to opposite spatial interference effects, with arrows producing faster RTs when the arrow direction was congruent with its position (a typical spatial Stroop effect), and eye gaze producing faster RTs when it was incongruent (a “reversed” spatial Stroop effect).
Moreover, consistent with previous studies, responses in general were slower for gaze than for arrow stimuli (Hietanen, Nummenmaa, Nyman, Parkkola, & Hämäläinen, 2006; Vlamings, Stauder, van Son, & Mottron, 2005). This suggests, presumably, that the coding of eye-gaze stimuli took more time than the coding of arrow stimuli. From our point of view, the slowing of RTs observed for gaze stimuli may have been due to their social significance and complexity, that induces a greater exploration of it. Supporting this view, Vlamings and coworkers showed slower RTs after eye-gaze than after arrow stimuli only in typically developed individuals, but not in individuals with autism, who are generally understood to be impaired in social attention behavior (e.g., Leekam, Lopez, & Moore, 2000).
Nevertheless, the most important result was the opposite congruency effects observed for eye gaze and for arrows within the same task. This dissociation is difficult to reconcile with the domain-general view of attentional processes. The opposite congruency effects for gaze and arrows have been replicated in subsequent experiments in our laboratory (Roman-Caballero, Marotta, Martin-Arevalo, & Lupiañez, 2017), and other studies have found that the reversed congruency effect is modulated by the emotional expression of a face when the whole face instead of just the eyes is used as the target (Jones, 2015; Torres-Marín, Carretero-Dios, Acosta, & Lupiáñez, 2017), thus supporting the social nature of the effect.
The reverse congruency effect found with eye-gaze stimuli seems consistent with the idea that participants are especially fast when the target face seems to look directly at them. Indeed, it is important to note that when a gaze stimulus is presented on the left and looks to the right (i.e., an incongruent trial), it is looking to the center, in the direction appropriate to make eye contact with the participant. In contrast, if the target face is presented on the left and looks to the left (congruent trial), it is looking away from the participant. This difference between direct and averted gaze might underlie the reversion of the typical Stroop effect observed with eye gaze.
Indeed, a number of studies have shown that human observers are faster to detect a face (Senju, Kikuchi, Hasegawa, Tojo, & Osanai, 2008) or eyes (Conty, Tijus, Hugueville, Coelho, & George, 2006) when eye contact is maintained. Moreover, eye contact results in benefits in processing for other face-related features, such as the gender or the identity of the face (Senju & Johnson, 2009). Thus, eye contact could explain the “reversed” spatial Stroop effects observed in our and in previous studies (Cañadas & Lupiáñez, 2012), and would therefore be an essential social feature of the attentional mechanisms triggered by gaze cues, able to dissociate between gaze and arrow attention mechanisms.
Another explanation for the reversed congruency effect might be related to the “mentalizing” theory (Baron-Cohen et al., 1997), which refers to the human ability to determine another individual’s state of mind from their eye gaze. In particular, eye gaze may be used to perceive, explain, and anticipate the behavior and/or the intentions of others. Thus, rather than sharing mutual gazes in the incongruent condition, participant and stimulus may be engaged in a joint encoding of information about self and others’ attention; in other words, in incongruent trials, eyes presented on the left but looking to the right might be interpreted as having an intent to move to the right, so switching to this location might be an advantage, regarding the participant’s allocation of attentional resources (it is important to note that right-looking eyes required a right response). Consequently, perhaps for this reason, the discrimination of gaze direction was faster in the incongruent condition.
Although more research will be needed to clarify the nature of the reverse congruency observed with eye-gaze stimuli, both explanations seem coherent with the idea that another person’s eye gaze indicates more than just a direction; it provides a window into their intentions, and it can signal approach or avoidance (Hietanen et al., 2008). Arrows simply provide directional information and do not signal intent or the possibility of a social interaction. Probably this is the reason why a classical spatial Stroop effect was observed when arrows were used as the targets. The role of intent in gaze can also help explain the growing evidence of gaze-specific effects observed in the literature when a “qualitative” rather than a “quantitative” approach has been used to dissociate between gaze and arrow attentional mechanisms. In particular, these studies have focused on effects other than the usual facilitation effect produced by gaze and arrow cues, such as object evaluation (e.g., Bayliss et al., 2006), object selection (Marotta et al., 2012), long-term memory (Dodd et al., 2012), working memory (Gregory & Jackson, 2017), and spatial interference, as we studied here.
Our results constitute evidence against the domain-general view of attentional processing and support the notion that social attention may be special, since it is hard to imagine how the same attentional mechanism could produce opposite influences on spatial interference. However, it is possible that gaze and arrows may share some processes but not others. For example, in addition to the spatial interference effect, which might arguably be commonly produced by arrow and gaze stimuli, other processes, such as eye contact, would be specific to gaze stimuli. Such processes should work in opposition to the spatial interference effect, thus explaining the reversing of the observed spatial Stroop effect in the case of the eye-gaze stimuli. Therefore, on the one hand, it could be argued that gaze and arrows, as directional cues, may share a domain-general attentional process responsible for the results usually found in both the spatial-cueing (e.g., Birmingham & Kingstone, 2009) and perspective-taking (e.g., Santiesteban et al., 2014) literatures, showing similar effects for both cue types. On the other hand, other processes related to theory of mind and social interactions could be invoked to explain why eye-gaze stimuli produce different effects from arrows when the nature rather than the magnitude of the attentional effects is considered.
Future research using our paradigm with brain-imaging techniques could be very useful for providing more direct evidence regarding the underlying processes contributing to the observed dissociation. One possibility is that the brain areas related to the domain-general attentional process would be involved with both arrows and gaze stimuli, whereas other brain circuits related to theory of mind and eye contact, such as the superior temporal sulcus, would be especially involved with eye-gaze stimuli.
Notes
The face stimulus was drawn from the MacBrain Face Stimulus Set, developed by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham, at tott0006@tc.umn.edu, for more information concerning the stimulus set.
References
Baron-Cohen, S., Wheelwright, S., & Jolliffe, A. T. (1997). Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger syndrome. Visual Cognition, 4, 311–331.
Bayliss, A. P., Paul, M. A., Cannon, P. R., & Tipper, S. P. (2006). Gaze cuing and affective judgments of objects: I like what you look at. Psychonomic Bulletin & Review, 13, 1061–1066.
Birmingham, E., & Kingstone, A. (2009). Human social attention. Annals of the New York Academy of Sciences, 1156, 118–140.
Cañadas, E., & Lupiáñez, J. (2012). Spatial interference between gaze direction and gaze location: A study on the eye contact effect. Quarterly Journal of Experimental Psychology, 65, 1586–1598. doi:https://doi.org/10.1080/17470218.2012.659190
Conty, L., Russo, M., Loehr, V., Hugueville, L., Barbu, S., Huguet, P.,. . . George, N. (2010). The mere perception of eye contact increases arousal during a word-spelling task. Social Neuroscience, 5, 171–186.
Conty, L., Tijus, C., Hugueville, L., Coelho, E., & George, N. (2006). Searching for asymmetries in the detection of gaze contact versus averted gaze under different head views: A behavioural study. Spatial Vision, 19, 529–545.
Cousineau, D. (2005). Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorial in Quantitative Methods for Psychology, 1, 42–45.
Dalmaso, M., Galfano, G., & Castelli, L. (2015). The impact of same-and other-race gaze distractors on the control of saccadic eye movements. Perception, 44, 1020–1028.
Dodd, M. D., Weiss, N., McDonnell, G. P., Sarwal, A., & Kingstone, A. (2012). Gaze cues influence memory … but not for long. Acta Psychologica, 141, 270–275.
Driver, J., IV, Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6, 509–540.
Friesen, C. K., Ristic, J., & Kingstone, A. (2004). Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30, 319–329. doi:https://doi.org/10.1037/0096-1523.30.2.319
Frischen, A., Bayliss, A. P., & Tipper, S. P. (2007). Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin, 133, 694–724. doi:https://doi.org/10.1037/0033-2909.133.4.694
Funes, M. J., Lupiáñez, J., & Milliken, B. (2007). Separate mechanisms recruited by exogenous and endogenous spatial cues: Evidence from a spatial Stroop paradigm. Journal of Experimental Psychology: Human Perception and Performance, 33, 348–362. doi:https://doi.org/10.1037/0096-1523.33.2.348
Gregory, S. E., & Jackson, M. C. (2017). Joint attention enhances visual working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43, 237–249. doi:https://doi.org/10.1037/xlm0000294
Hietanen, J. K., Leppänen, J. M., Peltola, M. J., Linnaaho, K., & Ruuhiala, H. J. (2008). Seeing direct and averted gaze activates the approach–avoidance motivational brain systems. Neuropsychologia, 46, 2423–2430.
Hietanen, J. K., Nummenmaa, L., Nyman, M. J., Parkkola, R., & Hämäläinen, H. (2006). Automatic attention orienting by social and symbolic cues activates different neural networks: An fMRI study. NeuroImage, 33, 406–413.
Jones, S. (2015). The mediating effects of facial expression on spatial interference between gaze direction and gaze location. Journal of General Psychology, 142, 106–117.
Kuhn, G., & Benson, V. (2007). The influence of eye-gaze and arrow pointing distractor cues on voluntary eye movements. Perception & Psychophysics, 69, 966–971.
Kuhn, G., & Kingstone, A. (2009). Look away! Eyes and arrows engage oculomotor responses automatically. Attention, Perception, & Psychophysics, 71, 314–327. doi:https://doi.org/10.3758/APP.71.2.314
Leekam, S. R., Lopez, B., & Moore, C. (2000). Attention and joint attention in preschool children with autism. Developmental Psychology, 36, 261–273.
Lu, C.-H., & Proctor, R. W. (1995). The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychonomic Bulletin & Review, 2, 174–207. doi:https://doi.org/10.3758/BF03210959
Macrae, C. N., Hood, B. M., Milne, A. B., Rowe, A. C., & Mason, M. F. (2002). Are you looking at me? Eye gaze and person perception. Psychological Science, 13, 460–464.
Marotta, A., Lupiáñez, J., Martella, D., & Casagrande, M. (2012). Eye gaze versus arrows as spatial cues: Two qualitatively different modes of attentional selection. Journal of Experimental Psychology: Human Perception and Performance, 38, 326–335. doi:https://doi.org/10.1037/a0023959
Pires, L., Leitão, J., Guerrini, C., & Simões, M. R. (2017). Cognitive control during a spatial Stroop task: Comparing conflict monitoring and prediction of response-outcome theories. Acta Psychologica. Advance online publication. doi:https://doi.org/10.1016/j.actpsy.2017.06.009
Ricciardelli, P., Bricolo, E., Aglioti, S. M., & Chelazzi, L. (2002). My eyes want to look where your eyes are looking: Exploring the tendency to imitate another individual’s gaze. NeuroReport, 13, 2259–2264.
Roman-Caballero, R., Marotta, A., Martin-Arevalo, E., & Lupiañez, J. (2017, September). Las flechas no te miran: Diferencias cualitativas en los mecanismos atencionale inducidos por la mirada y flechas. Paper presented at the Reunión Científica sobre Atención (RECA 11) conference, Baeza, Spain.
Santiesteban, I., Catmur, C., Hopkins, S. C., Bird, G., & Heyes, C. (2014). Avatars and arrows: Implicit mentalizing or domain-general processing? Journal of Experimental Psychology: Human Perception and Performance, 40, 929–937. doi:https://doi.org/10.1037/a0035175
Senju, A., & Johnson, M. H. (2009). The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences, 13, 127–134.
Senju, A., Kikuchi, Y., Hasegawa, T., Tojo, Y., & Osanai, H. (2008). Is anyone looking at me? Direct gaze detection in children with and without autism. Brain and Cognition, 67, 127–139.
Tipples, J. (2008). Orienting to counterpredictive gaze and arrow cues. Perception & Psychophysics, 70, 77–87. doi:https://doi.org/10.3758/PP.70.1.77
Tomasello, M. (1995). Joint attention as social cognition. In C. Moore & P. J. Dunham (Eds.), Joint attention: Its origins and role in development (pp. 103–130). Hillsdale, NJ: Erlbaum.
Torres-Marín, J., Carretero-Dios, H., Acosta, A., & Lupiáñez, J. (2017). Eye contact and fear of being laughed at in a gaze discrimination task. Frontiers in Psychology, 8, 1954. doi:https://doi.org/10.3389/fpsyg.2017.01954
Vlamings, P. H., Stauder, J. E., van Son, I. A., & Mottron, L. (2005). Atypical visual orienting to gaze-and arrow-cues in adults with high functioning autism. Journal of Autism and Developmental Disorders, 35, 267–277.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marotta, A., Román-Caballero, R. & Lupiáñez, J. Arrows don’t look at you: Qualitatively different attentional mechanisms triggered by gaze and arrows. Psychon Bull Rev 25, 2254–2259 (2018). https://doi.org/10.3758/s13423-018-1457-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-018-1457-2