Abstract
Same/different concept learning has been demonstrated in previous research in rats using matching- and non-matching-to-sample procedures with olfactory stimuli. In Experiment 1, rats were trained on the non-matching-to-sample procedure with either three-dimensional (3D plastic objects; n = 3) or olfactory (household spices, n = 5) stimuli, then tested for transfer to novel stimuli of the same, and then the alternate, modality. While all three rats trained with olfactory stimuli showed generalized non-matching to novel odors, only one rat learned the 3D relation and showed generalized transfer to novel objects. Importantly, in this rat the 3D non-matching relation then immediately transferred to odors. In contrast, rats trained with scents did not show transfer to novel 3D stimuli until after training with one or two 3D stimulus sets. In Experiment 2, four rats were trained on an incrementing non-matching-to-sample task featuring 3D plastic objects as stimuli (3D Span Task). Responses to session-novel stimuli resulted in reinforcement. Only two rats learned the 3D Span Task; one rat performed with high accuracy even with up to 17 session-novel objects in a session. While these findings emphasize the exceptional olfactory discrimination of rats relative to that with 3D/tactile/visual cues, they also show that relational learning can be demonstrated in another modality in this species. Further, the present study provides some evidence of cross-modal transfer of relational responding in rats.
Similar content being viewed by others
Introduction
The ground-breaking work of Sarah T. Boysen in the field of comparative cognition illustrates the importance of studying relational learning and abstraction in non-human animals. In particular, Boysen notes that species and individual differences, appropriate and innovative methodologies, and alternative explanations for stimulus control of apparently relational or conceptual learning must be considered (Boysen, 1996; Boysen et al., 1996).
Same/different and identity/oddity relational learning have been demonstrated in a variety of animals and provide a foundation for the emergence of conceptual behavior across species. Matching- (MTS) and non-matching-to-sample (NMTS) tasks, as well as same/different procedures, are typically used to evaluate these relations. In MTS, presentation of a sample stimulus is followed by presentation of two or more comparison stimuli; one of these is physically identical to the sample and selection results in reinforcement. In the NMTS version, responses to the comparison different from the sample are reinforced. In same/different procedures, two separate responses are available to the animal; on trials when the sample and comparison stimuli are the same, responding on the “same” lever or response key is reinforced, but when sample and comparison differ, responding on the “different” lever or response key produces reinforcement (Daniel et al., 2015). In all cases, the correct response depends on the relation between the sample and comparison stimuli.
It is possible that animals may be quite accurate on these procedures without learning abstract relations due to learning stimulus configurations or stimulus-specific relations (see Berryman et al., 1965; Carter & Werner, 1978; Katz et al., 2008; McIlvane, 2013). Thus, a standard definition of abstract concept learning must include responding similarly to members of one stimulus class and differently to members of another, as well as transfer of accurate responding to novel members of the class (Katz & Wright, 2006; Keller & Schoenfeld, 1950; Lazareva & Wasserman, 2008). Thus, generalization or transfer tests in which novel stimuli are presented are required to determine whether abstract-concept learning has occurred. Furthermore, Katz and Wright (2006) noted that transfer test performance should be equal to or better than the accuracy on baseline training trials to demonstrate conceptual behavior. Using these approaches, evidence of identity/oddity concept learning has been shown in a variety of species including marine mammals (Herman et al., 1989; Kastak & Schusterman, 1994; Scholtyssek et al., 2013), apes (Oden et al., 1988; Thompson et al., 1997; Vonk, 2003) and other non-human primates (Brino et al., 2014; D’Amato et al., 1985; Katz et al., 2002; Wright et al., 2003), rats (Bruce et al., 2018; Galizio et al., 2018; Lazarowski et al., 2019; Peña et al., 2006), dogs (Lazarowski, et al., 2021), pigeons and other birds (Bodily et al., 2008; Magnotti et al., 2015; Wilson et al., 1985; Wright et al., 1988; Wright et al., 2016), echidna (Russell & Burke, 2016), and honeybees (Giurfa et al., 2001, but see Brown & Austin, 2021).
Particular features related to stimulus control and the development of relational learning are important to note, none more critical than stimulus presentation modality. As observed by Katz et al. (2007) and Slotnick (2001), species rely on different sensory receptors to solve complex problems. The importance of modality can be illustrated in studies on rats related to discrimination, reversal, and concept learning. Historically, several modalities have been used with rats in these procedures, including olfactory (e.g., April et al., 2011; Lazarowski et al., 2019; Lu et al., 1993; Pena et al., 2006; Prichard et al., 2015; Thomas & Noble, 1988), 2D visual (e.g., Iversen, 1993, 1997), three-dimensional (3D) visual/tactile/haptic (e.g., Denny et al., 1989; Mumby et al., 1990; Nakagawa, 1993; Rothblat & Hayes, 1987; Taniuchi et al., 2017; Tran et al., 1994), and auditory (e.g., Dube et al., 1993). Examples of these are described below.
Stimulus modality and matching or non-matching in rats
Iverson (1993, 1997) trained rats on a match-to-sample task using two visual stimuli, either steady or blinking lighted nose poke keys, in a fully automated MTS procedure. In the first experiment, results showed configural rather than relational learning; rats apparently learned the four stimulus configurations possible with two different stimuli. In the follow-up experiment, configurations were changed to see if the rats would respond instead based on the relation between stimuli rather than stimulus arrangements. So, instead of the sample always being in the center, it occurred randomly on any of the three keys. Next, the sample occurred always on the leftmost key. Both of these changes disrupted performance, and Iverson (1997) concluded that rats had learned to respond to specific configurations involving visual stimuli and their locations, but not abstract same/different concepts.
Rats were more accurate on a delayed non-match-to-sample task with visual stimuli when those stimuli were 3D objects that could also be manipulated (Rothblat & Hayes, 1987; Mumby et al., 1990). Both Rothblat and Hayes (1987) and Mumby et al. (1990) trained rats on a delayed NMTS task with 3D, unique stimuli in a test of working memory rather than generalized identity/oddity. Pairs of stimuli were randomly selected from a pool of 250–350 small “junk” objects and animals were tested using a sample runway and comparison runway. Rats were trained to respond to a sample for a food reward, then were allowed access to the comparison runway to select the non-matching object after different delays. Using delays of 10, 30, or 120 s, Rothblat and Hayes (1987) reported that group performance stabilized at about 75% at the 10-s delay, but accuracies dropped with the 30- and 120-s delays. Mumby et al. incorporated delays of 4, 15, 30, 60, 120, and 600 s. Rats were highly accurate with delays of 4 and 15 s (90%). With longer delays, accuracy was significantly depressed at first, but as the rats were tested for more sessions, accuracy improved for delays up to 120 s. However, not all of the 14 rats tested met mastery criteria, especially at the 120-s delay. In both of these studies, it was clear that rats learned non-match-to-sample with 3D stimuli but, as no novel transfer tests were conducted, critical evidence of generalized non-matching was not available.
Using olfactory stimuli has produced especially promising results using both manual and automated procedures. Pena et al. (2006) used a simultaneous MTS procedure in which rats were trained to dig in cups of sand that had been scented with household spices. Four rats were first manually presented with two olfactory stimuli on a tray in a modified operant chamber. Once they were responding at 90% accuracy in the MTS procedure, two new olfactory stimuli were added. On the first day that new scents were added to training, probe trials were assessed for generalized transfer. On those trials, odorants would appear as either novel samples or as novel incorrect comparisons. Thus, there were two types of probe trials (designated Novel Probes and Novel Combinations). By the end of the experiment, the rats had encountered between 21 and 35 total stimuli and were showing evidence of generalized identity matching. One limitation of the procedure was that the number of exemplars required for generalized identity to emerge could not readily be determined.
Using a similar manual procedure, Lazarowski et al. (2019) trained rats on either MTS or NMTS tasks in a set-size expansion study; they were initially trained using either a set of two or a set of ten olfactory stimuli, plastic Plexiglas lids that had been scented with household spices. Rats were required to push back the lid and retrieve a food reward. Once the subjects met criterion, they were given transfer tests (probes) with ten novel olfactory stimuli. After this probe, they received further training with the novel stimulus set until meeting the same criterion again. This meant that the group that previously had been trained with two stimuli was now being trained with ten stimuli (12 total exemplars) and the group originally trained with ten now had 20 exemplars. Upon meeting criterion, rats received a second transfer test with ten more novel stimuli. Results demonstrated the importance of number of training exemplars for generalized identity and oddity. The rats originally trained with ten stimuli did significantly better on the first transfer test than the group trained with two stimuli. Further, when the set size was expanded for the rats originally trained with two stimuli, they did significantly better on the second transfer test, so much so that no significant differences were found on the second transfer test between the group initially trained with two stimuli and the group initially trained with ten stimuli. In conclusion, Lazarowski et al. (2019) showed evidence of both generalized identity matching and non-matching given a sufficient number of exemplars.
Further, April et al. (2011) demonstrated that rats could learn generalized matching and non-matching with olfactory stimuli in manual procedures that utilized a reversal design. In Experiment 1, six rats were trained on either an MTS or an NMTS procedure with five odorants. They were tested in a modified operant chamber that allowed insertion of a sample tray with a cup filled with odorized play sand and then a comparison tray with two comparison stimuli (one matching and one non-matching). Rats were required to dig in the sand to obtain a food reward. Once they were accurate with five stimuli, a transfer test was conducted with five novel scents. There was clear evidence of transfer for three rats and considerable savings and rapid learning for the other three. The rats were then trained on the novel scents to criterion and then the contingencies were reversed on the second transfer test with five new olfactory stimuli. All rats responded consistently with the originally trained relation on the initial trials with new stimuli. In Experiment 2, April et al. (2011) tested nine rats on a similar MTS procedure but in an open field arena so that the rats could independently navigate to the stimulus cups. Five of the rats showed evidence of transfer to new odors when they were introduced.
In addition to these manual procedures, identity and non-matching have also been demonstrated using an automated olfactometer. Lu et al. (1993) trained rats with a go/no-go matching task that involved successive presentation of odorants (e.g., amyl acetate, butanol, ethyl acetate) in a nose poke port. Three rats learned both two- and three-odor matching to sample and showed evidence of rapid learning of two novel scents (linalyl acetate, geraniol). Because only average session accuracies were presented rather than first responses to the novel odorants, generalized identity could not be assessed.
Extending this research, Prichard et al. (2015, Experiment 2) tested six rats on four identity relations in an automated olfactometer using successive presentation of stimuli (e.g., bubblegum, apricot, root beer) in a go/no-go procedure. Prichard et al. (2015) used discrimination indices to compare responses on “positive” and “negative” trials that consisted of a sample odor followed by either a matching (positive) or a non-matching (negative) comparison. Responses to the matching odor were reinforced. Once rats were showing strong discrimination, four novel stimuli were introduced as unreinforced probes to test for transfer. Response rates were high on identity probe trials (positive) and low on non-matching (negative) probe trials for five of the six rats. The similar patterns of responding on baseline and probe trials that were shown by most rats provided a demonstration of generalized identity matching.
Bruce et al. (2018) also used an olfactometer to present odor stimuli to rats. In this study, ten rats were assigned to either matching or non-matching procedures, using the same go/no-go procedures as in Prichard et al. (2015). Rats were initially trained with four odorants and once they were showing strong discrimination, four new odorants were presented in transfer tests on unreinforced trials. Again, comparison of response rates on positive and negative probe trials was used to assess generalized matching or non-matching. Another test for transfer to novel stimuli was introduced after training with the novel scents, followed by training on that set of odors. Then, as in April et al. (2011), contingencies were reversed, and four new odors were introduced as probes. Rats trained on MTS showed clear evidence of generalized matching on both transfer tests with discrimination indices similar to those obtained during baseline. Rats trained on NMTS showed less complete transfer to new stimuli in the first transfer phase, although they showed stronger transfer on the second. In the reversal phase, there was a dramatic drop in accuracy on the first day of reversed contingencies in both groups, consistent with the rats’ responding based on the relation from the previous testing session.
Cross-modality transfer or rapid learning?
The majority of experiments studying conceptual behavior in non-humans have used stimuli on novel transfer tests that are from the same modality (e.g., visual, olfactory, auditory, etc.) as the training stimuli. Because the training and testing stimuli are from the same modality, it has been argued that some form of stimulus generalization is controlling responding in these experiments, rather than transfer of relational control (Mackintosh, 2000). Mackintosh argued that stimuli from the same modality will share common properties and that probe trials from the same modality used in training cannot be said to be truly novel tests of relational concept learning. While a meta-analysis done by Wright and Katz (2007) suggested that the generalization hypothesis is an unlikely explanation, they did note that increasing the number of stimuli used in training compounds increases the possibility that testing stimuli will not be truly novel. At the least, same-modality transfer tests restrict inferences about identity/oddity concept learning to that modality. For example, MTS and NMTS training with olfactory stimuli in rats results in generalized matching and non-matching to novel odors, but whether the identity concept is extended to other modalities is unknown.
To date, attempts to study cross-modal concept learning are limited. In some studies, the overall modality is the same (e.g., visual) but different dimensions of the modality are trained and tested. For example, Scholtyssek et al. (2013) reported transfer of same/different relational learning across visual dimensions in a harbor seal. The seal was originally trained using white, two-dimensional shapes in a same/different, go/no-go testing paradigm. If the shapes were the same, the seal remained at a stationary target for 5 s (no-go) and if the shapes were different, the seal touched the monitor screen within 5 s of stimulus presentation (go). Scholtyssek et al. (2013) used multiple exemplar training with set-size expansion to train different same or different combinations of two, then four, then 15 different two-dimensional shapes. After the seal showed high accuracy with these trained pairs, novel shapes were used in combinations for unique transfer tests (120 unfamiliar pairs of two-dimensional stimuli) and the seal scored over 80% correct. In the last phase of the study, the experimenters tested for transfer to the extra-dimensional properties of brightness and pattern. In the first extra-dimension transfer test, two-dimensional shape pairs of stimuli were intermixed with pairs of visual stimuli that differed in brightness rather than shape and the seal spontaneously showed accurate (> 80%) responding to these stimuli. Pattern (e.g., crosshatch) differences were then added and pairs of stimuli differing in shape, brightness, or pattern were presented randomly. Again, the seal responded consistently with same/different relational learning to these novel stimuli.
Similarly, Truppa et al. (2010) trained six tufted capuchin monkeys on a simultaneous MTS procedure with white, two-dimensional shapes presented on a computer monitor. In Experiment 1, monkeys were presented with a sample shape that remained on the computer monitor when two comparison shapes were presented. The monkeys were required to touch the comparison that matched the sample to receive a food reward. Initial training and tests for transfer involved a set of 22 different white shapes. Once the monkeys were responding accurately, Truppa et al. (2010) tested them with Set 2, 200 stimuli that were presented in a single 100-trial transfer test. Three of the six monkeys performed above chance. Because the experimenters were concerned that initial training involved too few exemplars, in Experiment 2 the same monkeys received further training with Set 2. Once they were responding with 80% accuracy, they were tested with Set 3, 200 new stimuli that were presented in pairs that again differed only in shape. All six subjects showed significantly above chance performance (all over 70%). Truppa et al. (2010) then tested the monkeys in a single 96-trial session that featured pairs of circles (one shape) in four colors (white, gray, yellow, and blue). All color combination pairs were tested in four blocks of 24 unique trials. Four of the monkeys performed above chance in the first block. Next, the monkeys were given a single 96-trial session featuring six white shapes (e.g., circle, square, triangle, diamond, arrow, and pentagon) that differed in size (large and small). Again, unique pairs were presented in blocks in the transfer test, and two animals performed above chance on the first block. In both the color-only and size-only transfer tests, all monkeys performed well above chance (all over 75%) on these sessions, demonstrating rapid acquisition of the relation with the new visual dimension.
In contrast to Truppa et al. (2010), D’Amato et al. (1985) did not find evidence of transfer in eight capuchin monkeys trained on an MTS procedure using two dimensional lights as stimuli. Each monkey was initially trained on a different combination of two of the stimuli (e.g., illuminated red dot, square, inverted triangle, etc.). Once they met mastery criterion for one pair (e.g., over 70%), an additional pair of stimuli were added. Of the eight monkeys, four showed immediate transfer from the initial two exemplars to the next two novel stimuli. Three more monkeys showed transfer by the third introduction of new stimuli. Four of the monkeys moved on to Experiment 2, in which D’Amato et al. tested for cross-modal transfer to steady versus flashing green samples; however, the monkeys did not show transfer of the generalized identity relation. The authors concluded that the small number of exemplars in Experiment 1 may have hindered generalized matching across visual dimensions.
An example in which the training and testing modalities were qualitatively different is a study on one bottlenose dolphin, and she required extensive pretraining (Herman et al., 1989). In this experiment, the dolphin first learned to associate a series of underwater whistle sounds with objects located in her tank, actions, and locations. Next, she underwent training to learn to associate a visual replica of the object held by the trainer with an object in her tank. An MTS procedure was used in training, and it was initially necessary to use auditory and visual cues together as a compound stimulus. The whistle was faded out, and the dolphin eventually achieved 80% or higher mastery on the match to sample trials. In Experiment 2, novel transfer tests were applied. There were three different types of tests: (1) objects that had previously been trained with an acoustic cue, (2) objects that were familiar to the dolphin but did not have an acoustic cue, and (3) objects that were completely novel. During all types of probes trials, two objects were placed in the tank. Again, the dolphin was shown the object, heard an action sound, and received a food reward for performing the appropriate object/action pair. She showed high levels of accuracy for all twelve probe trials. Experimenters argued that this was evidence for cross-modal matching because the visual object and auditory action cues were used to create one command that included elements from two modalities. However, the extensive pretraining with objects and sounds that the subject was familiar which makes a case that this may not be true generalized matching. While the dolphin did learn to transition from using auditory samples to visual samples, the comparison stimuli were always of the same modality.
Denny et al. (1989) reported some evidence of rapid learning across modalities (black/white and haptic) for some albino rats tested in a Y-maze using an MTS task. The stem and the arms of the Y maze were constructed such that they could be either black or white. Further, there were colored hurdles for the rat to navigate before getting to the arms; these matched the color of the stem. For some rats, a differential outcomes procedure was used such that different numbers of food pellets were used for choosing the black and white correct comparisons. Only half of the six rats could learn the initial two-exemplar color discrimination. One rat was trained first on the haptic discrimination (hardware cloth or smooth flooring in the stem and arms) and reached criterion. All four of these rats had experienced differential outcomes. For these four rats, delays of 0, 2, 4, 8, or 12 s were implemented between sample and comparisons by inserting a “shield” the same color as the stem in front of the comparisons that was lifted when the delay was up. As expected, accuracy decreased as the delay increased. Then, the experimenters tested for transfer to a new modality. Two of these four rats showed rapid learning of the new discrimination, though not immediate transfer on the first stimulus presentation in the new modality.
Experiment 1
Given the limited studies on cross-modal transfer as evidence of generalized identity and oddity, we designed Experiment 1 to test for transfer across qualitatively different modalities, rather than stimulus dimensions, in an NMTS procedure with rats. Examining whether there are differences in acquisition and generalization across multiple modalities would shed light on the flexibility of relational learning especially related to its ecological validity as an adaptation. Building on the clear demonstration of generalized identity and/or non-matching in rats using olfactory stimuli (e.g., Bruce et al., 2018; Lazarowski et al., 2019), we trained one group of rats using this modality, then tested for transfer to three-dimensional objects. Another group of rats were trained on 3D objects, then tested for generalized non-matching in the same modality, and next for transfer to the olfactory stimuli. Noting the importance of multiple exemplars in training, we used ten exemplars (cf. Lazarowski et al., 2019) for each training set. We expected that rats would show transfer of relational control to novel stimuli in the original training modality and to stimuli in a different modality. Further, given rats’ exceptional olfactory discrimination, we predicted that rats trained with olfactory stimuli would require fewer sessions to reach their first transfer test compared to the number of sessions required for rats trained initially on 3D stimuli.
Method
Subjects
Eight male Sprague Dawley rats, approximately 60–90 days old at the start of testing, were used in this experiment. Rats were housed in individual home cages as part of a colony maintained on a 12-h reverse light/dark cycle. Rats were tested 5 days a week during the dark cycle. Outside of the experimental session, rats were given free access to water and maintained at approximately 85% of free-feeding body weight. The subjects received their daily food ration, in the form of Purina™ Lab Diet grain pellets, approximately 30 min after the end of the experimental session.
Apparatus
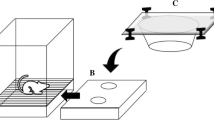
The experimental chamber was a modified operant chamber (30 cm × 33.75 cm × 25 cm); the top, front, and back of the operant chamber were clear Plexiglas, while the left side, right side, and floor were metal. The floor consisted of 14 metal rods spaced 1.25 cm from each other and the walls of the chamber. There was a 10 cm × 30 cm gap at the bottom of the front wall of the chamber to allow a stimulus tray to be manually inserted into the chamber.
As seen in Fig. 1, stimuli were presented by the experimenter on two trays constructed of clear Plexiglas, the sample tray and the comparison tray (each 25 cm × 20 cm × 3.75 cm). Circular holes in the trays contained plastic 2-oz condiment cups filled approximately 75% with sand. The sample tray (Fig. 1A) contained one hole in the center and comparison tray (Fig. 1B) contained two holes 3.25 cm from the sides of the tray, with 7.8 cm between holes. Surrounding each hole were four metal screws used to guide a plastic stimulus lid straight back when it was pushed by the rat (see Fig. 1 in Lazarowski et al., 2019, for an illustration).
Stimuli
Plexiglas lids (5 cm × 7.5 cm) were used to deliver stimuli. Two types of stimuli were used for this experiment, olfactory and 3D. Olfactory stimuli were created by scenting sets of the Plexiglas lids with common household spices or oils obtained from Great American Spice Company™ (e.g., cumin, rosemary, oregano, raspberry, etc.). To ensure that the lids absorbed the scent, they were housed in containers containing the odorant for a minimum of 1 week and at all times not in use. There was a pool of 40 scents, assigned to four unique sets of ten (see Table 1).
The 3D stimuli (see Fig. 1C and 1D) were constructed from Legos™ and varied in color and shape. The 3D objects were mounted onto unscented Plexiglas lids described above using Velcro (see Fig. 1C). Duplicates of all stimuli were used throughout the study to control for scent marking cues. Within each experimental session, each member of a pair of duplicates could serve as both the sample and comparison stimulus. Forty different stimuli were constructed and then divided into four sets of 10 stimuli (plus their duplicates) that were stored together in plastic containers when not in use.
Procedure
Shaping
Figure 2 presents an overview of all steps in the procedure of Experiment 1. Each rat was acclimated both to the experimenter and the operant chamber before beginning the experiment. First, 45-mg sucrose pellets (BioServ) were given to each rat daily in their home cage until all pellets were readily consumed. The next day rats were habituated to the experimental chamber for approximately 15 min. On the following session, rats were presented with the sample tray with an open cup of sand that had a sucrose pellet on top of the sand. After the rats reliably retrieved the pellet, the experimenter shaped digging behavior by burying the pellet in slightly greater increments each time.
Typical procedure for subjects in Experiment 1
Once the rats reliably retrieved the pellets from a depth at which they were completely covered by sand, the experimenter then presented the comparison tray with a pellet slightly buried in each cup. As with the sample tray, the pellets were first presented above the sand and were buried more each trial until fully-buried pellets from both cups were reliably retrieved. Once the pellets were completely buried and retrieved, then only one cup was baited. After the rat learned to check both cups and retrieve a pellet from one, lids were introduced. These lids were opaque, with no scent and no object. On the first of these trials, the lid was stationed behind the cup, covering none of its surface. Lids were then stationed over the cup at greater amounts until completely covering the cup. Shaping continued in this way until the rats reliably pushed the lid and retrieved the pellet. Then, the rats were given mock trials. During a mock trial, the sample tray was presented and followed by the comparison tray, contingent upon retrieval of a pellet in the sample cup. After pellets were reliably retrieved from both sample and comparison cups, the experiment proper began.
Non-match-to-sample (NMTS) training
Rats were assigned to NMTS training using one of the sets of ten stimuli – either olfactory or 3D. Five rats were assigned to 3D stimuli for training and three to the olfactory stimuli. A trial began with the insertion of the sample tray into the chamber. After the rat pushed back the lid and dug in the sand, the comparison tray was presented, and the rat was given 2 s to observe the comparisons. During this time, the comparison tray was held just outside the chamber where the rat could see and smell but not touch the stimuli. One comparison always matched the sample (S-) and one did not (S+). After 2 s, the experimenter slid the comparison tray into the chamber. Both comparison cups were baited with a sucrose pellet to ensure that rats were not relying on tracking the scent of the pellet. If the rat pushed the non-matching lid (S+) aside, he was allowed 2 s to retrieve the pellet and then the comparison tray was removed. If a response was first made to the S-, the comparison tray was immediately removed from the chamber. That trial was repeated for a maximum of three times until a correct response was made, however, only the first trial presentation was used in calculating session accuracy and rats rarely required more than one repeated trial. Throughout the study, a response was defined as movement of the plastic lid with either the paws or nose such that the lid was pushed back over the cup past the first set of screws on the tray. Trials continued in this way until the rats reached a criterion of 90% correct (27 out of 30 trials) on one session. The intertrial interval was 30 s.
During all training sessions, each stimulus appeared as the sample/incorrect comparison and correct comparison three times. Location of the correct comparison was counterbalanced between the right and left positions on the tray. Trials were also balanced such that the correct comparison was never located on any one side more than two trials in a row. Also, the same stimulus was not correct on more than two consecutive trials and no stimulus appeared as the sample on more than two consecutive trials. If a stimulus was used twice in a row, the duplicate of the pair not used in the previous trial was used for the next trial.
Sample reinforcement reduction
After reaching the first training criterion, the probability that any sample was baited dropped from 100% to 75%. Criterion on this phase was 83% correct (25 out of 30 trials) for two consecutive days. When this criterion was met, the probability of any sample being baited dropped to 50%. Sample reinforcement rate remained at this level for the remainder of the study (cf. Pena et al., 2006). To gain access to the comparison tray, the rats were still required to dig in the sample even though it contained no pellet. Sample reinforcement reduction was important because on transfer probe trials there was no sample reinforcement. This was done to rule out the possibility that the rats were responding based on which stimulus had been most recently reinforced.
Novel modality exposure
After meeting criterion in the 50% sample reinforcement phase, rats moved to the novel modality exposure phase to habituate the rats to stimuli from the other modality but without any direct training. Thus, in this phase, sessions consisted of trials with the set of ten stimuli used in training, as well as a set of ten stimuli from the alternate modality.
There were still 30 trials in a session; 20 of these trials were training trials. The remaining ten trials were novel modality exposure trials. These ten trials were divided into five sample trials and five comparison trials. On sample trials, only a sample stimulus was presented. On comparison trials, a pair of identical stimuli were presented together on the comparison tray. A response was scored as correct if the rat pushed the lid and retrieved the pellet under either stimulus. While the overall percentage of sample reinforcement remained at 50%, all novel modality exposure trials were reinforced.
The sessions were constructed such that the novel modality exposure trials did not occur consecutively. Sessions were divided into blocks of three such that there was one novel modality exposure trial and two NMTS training trials in each block. Sessions were constructed in this way to ensure that the subjects did not inadvertently learn a relation between sample exposure trials and comparison exposure trials, or exposure trials and training trials. Novel modality exposure sessions were conducted on two non-consecutive days a week during the 50% sample reinforcement phase. Criteria to advance were two consecutive days at 90% accuracy on the training trials and one day of 90% responding (nine out of ten) on novel modality exposure trials.
Original modality probe
The next session after meeting these criteria, the rats were exposed to Original Modality (OM) Probe 1 to test for generalization of the training contingencies to novel stimuli. For this probe session, the set of ten training stimuli were presented as well as a new set of 10 stimuli from the same modality. The session consisted of 30 trials; the first ten were baseline training trials. To receive novel probe trials, the rats were required to respond correctly to at least seven out of these ten trials. If a rat did not meet this criterion, he received training trials for the rest of the session rather than any probe trials and was required to meet criterion again before another probe session was conducted. If a rat did reach at least 70% accuracy on the first ten trials, the next 20 trials consisted of ten more training trials interspersed with ten trials using the novel stimulus set (i.e., ten probe trials). On probe trials all stimuli were drawn from the new stimulus set. Sample stimuli were not baited but both comparison stimuli were baited on probe trials to ensure that accurate performance was not based on detecting the sucrose reward. Each stimulus from the new set served as the sample/incorrect comparison once and the correct comparison once. Novel probe trials never occurred more than two times consecutively. Overall percent correct was recorded, as well as percent correct on novel probe trials.
After the OM Probe 1 session, rats were given further training on the new stimulus set until they reached a criterion of two consecutive days of 90% correct or better. Once criterion was met, they received the cross-modality probe session.
Cross-modality probes
The cross-modality probe session consisted of 40 trials – 20 baseline and 20 probe trials. Ten of the probe trials used novel stimuli from the originally trained modality (OM Probe 2). Ten probe trials used stimuli from the second, untrained modality (Cross Probe 1). The remaining 20 trials were baseline training trials. As in original modality probe sessions, the first ten trials were used to assess readiness for probe trials; 70% or better accuracy was required for the probe session to continue. The other ten baseline trials were interspersed between probe trials. Again, trials of any one type did not occur more than twice consecutively. Overall percent correct was obtained, as well as percent correct on both novel probe trial groups.
Sessions after the cross-modality probe were used for further training on the set of new modality stimuli that had just served as probes. After meeting criterion on this phase (two consecutive days of 90% or better accuracy), subjects received a final probe in the new modality.
Cross Probe 2 proceeded as the other probe sessions; rats were required to achieve at least 70% accuracy on the first ten baseline training trials. The subsequent 20 trials consisted of ten training trials interspersed with ten novel probe trials from the new modality. After the final probe, the rats received an additional day of training with stimuli from the set used on the final probe.
Data analysis and inter-rater reliability
Performance on novel probes for each subject was tested for statistical significance using binomial tests. Significantly high performance on novel probes suggested that the behavior of the rats had come under the control of the relation between stimuli used in training. Further, accurate performance on the cross-modality probe would indicate that this control was generalized to stimuli from an untrained modality.
To assess reliability in scoring, videos from all probe sessions for each rat and ten training trials picked at random for each rat were independently scored. Of the 540 total trials compared, experimenters agreed on the rat’s choice of comparison stimuli on 100% of the novel probe trials and 98% on baseline trials.
Results and discussion
All three rats trained first with olfactory stimuli (A5, X5, and D4) learned the NMTS procedure and were tested on both original modality and cross-modality probes. However, only one (Z11) of the five rats originally trained with 3D stimuli learned the NMTS procedure. Performances for the four rats that received probes are shown in Figs. 3, 4, 5 and 6. In each figure, closed circles represent percent correct on baseline sessions, open squares represent performance on original modality probes, and closed triangles represent Cross-modality probes. Sample reinforcement reduction phases are also shown on each figure separated by vertical lines.
Initial NMTS procedure with olfactory stimuli
As seen in Fig. 3, A5 required 55 sessions to complete the sample reinforcement reduction phases, including exposure to novel modality stimuli with a set of ten 3D stimuli. After meeting criterion, A5 received the first olfactory probe session (OM Probe 1, open square) and scored 80% correct (binominal test, p = .055). The rat then was trained with the ten new olfactory stimuli and required five days to meet criterion for the Cross-Modality Probe. During this probe session, A5 showed generalization to ten more new olfactory stimuli (OM Probe 2; 90% correct, binominal test, p = .011) but did not show transfer to 3D stimuli (Cross Probe 1; 60% correct, ns). After 25 sessions of training with the 3D stimuli, A5 met criterion for a second 3D probe session (Cross Probe 2), and his performance was again 60% on ten new 3D stimuli. We were able to give A5 additional NMTS training with these new 3D stimuli on the NMTS procedure, and after seven sessions, he reached criterion for another 3D probe. On this probe (Cross Probe 3), he was 80% correct on probe trials (binominal test, p = .055).
Figure 4 shows that Subject D4 reached criterion for the first olfactory probe after 41 sessions of training including sample reinforcement reduction and novel modality exposure. On OM Probe 1, he failed to respond within 120 s on the first four presentations of new stimuli, but then scored 83% on the remaining six probe trials (5/6 correct, binomial test, p = .11). We discovered that he was averse to one of the new scents (nutmeg) and would not respond to it; we substituted a new scent (sassafras) and incorporated that into the training set. He then required 15 NMTS sessions with the ten new olfactory stimuli to meet criterion for the Cross Modality Probe. On this probe session, D4 scored 80% correct on another new set of olfactory stimuli (OM Probe 2; binomial test, p = .055) but only 50% on new 3D stimuli (Cross Probe 1, ns). D4 then required only 13 NMTS sessions with the new 3D stimuli to reach criterion for a second 3D probe. On Cross Probe 2, D4 scored 90% correct with a new set of 3D stimuli (binomial test, p = .011).
Subject X5 had originally received NMTS training and two probe tests (all with sets of ten novel olfactory stimuli) in a previous experiment (Lazarowski, et al., 2019). Thus, he began the current study on his 63rd testing session. This meant that this rat had three (not just two) olfactory probes and two 3D probes. As seen in Fig. 5, Subject X5 had required 56 sessions to complete the Sample Reinforcement Reduction phases to reach the first probe. He then required only two sessions to reach the second olfactory probe. Subject X5 scored 90% correct on both his first and second OM olfactory probes (binomial tests, p = .011).
Upon starting the current experiment, X5 experienced 12 baseline sessions with the probe 2 stimuli, as well as novel exposure trials, and met criterion for a Cross Modality Probe session. His transfer to new olfactory stimuli remained high (OM Probe 3; 80% correct, binomial test, p = .055), but was at chance levels with 3D stimuli (50% correct, ns). However, after receiving 45 days of training with these 3D stimuli, he received a second 3D probe and achieved 90% correct (binomial test, p = .011).
Thus, rats originally trained with olfactory stimuli learned the non-matching task and then showed clear transfer to novel stimuli in the original modality probe tests. This is consistent with the results of Lazarowski et al. (2019) who found that rats trained on NMTS with 10 exemplars of olfactory stimuli learned the task in 14–57 sessions and then showed immediate transfer to new olfactory stimuli in probe trials. While rats in the present experiment did not show immediate transfer to the 3D modality in Cross Probe 1, they did show generalization to new stimuli by the second or third probes with 3D stimuli after NMTS training with that modality. Further, there was evidence for savings for learning the NMTS procedure with each new set of stimuli.
Initial NMTS procedure with 3D stimuli
Subject Z11 was the only subject to complete the experiment after receiving initial training with 3D stimuli; he required 91 sessions of NMTS training before reaching the first 3D probe (see Fig. 6). On this probe, he responded at 80% accuracy to ten new 3D stimuli (OM Probe 1, binomial test, p = .055). He then needed only five sessions of training with these new stimuli to meet criterion for a Cross-Modality Probe with olfactory stimuli. On the Cross-Modality Probe, Z11 scored 90% correct with both the new 3D (OM Probe 2) and the novel olfactory stimuli (Cross Probe 1; both binomial tests, p = .011). Thus, after NMTS training with only 3D stimuli, this rat showed generalized non-matching to a new modality without any explicit training with olfactory stimuli. He then reached criterion for a final probe session after 14 sessions of further training with olfactory stimuli. Z11’s generalization was strong on this probe as well, scoring 90% correct on a new set of olfactory stimuli (Cross Probe 2, binomial test, p = .011). Like the rats trained with olfactory stimuli first, this rat’s performance showed a savings that even transferred across modalities.
Four rats assigned to the 3D stimuli never reached criterion on the NMTS procedure and thus could not be tested with probes. Subject A8 reached the 50% Sample Reinforcement Reduction phase but then accuracy decreased, and after 140 sessions, he was dropped from the study; percent correct responding on his final 10 sessions was only 72%. Subject W26 completed 32 sessions of 100% and 63 sessions of 75% sample reinforcement. This rat’s responding was quite variable, with mean accuracy on the NMTS procedure during the final ten sessions of only 68%. Thus, he was dropped from the study after 95 sessions. Two subjects did not perform well enough to advance to sample reinforcement thinning. Subject A4 completed 75 sessions with 100% sample reinforcement with an average of only 70.5% during the last ten sessions. Finally, Subject D12 never reached mastery criterion in the 100% Sample Reinforcement Reduction phase following 75 sessions; the average of his last 10 sessions was 70%.
Savings across training sets and modalities
Table 2 shows the number of training sessions required between probes for all rats. Overall, the rats initially trained with olfactory stimuli seemed to show similar levels of responding. All three rats reached the first probe in 56 or fewer sessions. All three rats also showed generalization of relational control to stimuli within the same modality, scoring 80% or higher on their first probes. They then showed equal or improved performance on olfactory probes and chance performance on the first 3D probe. Alternatively, only one subject trained with 3D stimuli was able to reach mastery criterion and receive probes. It took this rat (Z11) nearly twice as long (91 sessions vs. 56 or less) to reach the first probe compared to those rats trained initially with olfactory stimuli. This longer acquisition is also different from the rats trained by Lazarowski et al. (2019) on the olfactory non-matching procedure. After meeting criterion, Z11 also showed generalized non-matching to novel stimuli from the trained 3D modality. Subject Z11 was unlike the other three rats, however, in that he also showed generalized non-matching with olfactory stimuli without explicit training. All rats seemed to transfer some learning across modalities. They were all able to show relational control with the new stimulus modality after only about half the number of required training sessions.
In summary, Experiment 1 provided evidence of generalized non-matching to sample after training with either olfactory or 3D stimuli. Cross-modal transfer of non-matching from the 3D modality to olfactory stimuli was also demonstrated in the one rat tested (Z11); in contrast, cross-modal transfer of non-matching from the olfactory modality to 3D stimuli failed to emerge in any of the rats.
It is worth noting that rats acquired the non-matching task more easily when the stimuli were olfactory rather than 3D. This was not surprising as numerous studies have shown superior learning and performance on a variety of complex tasks with olfactory stimuli in rats (Nigrosh et al., 1975; Slotnick, 2001). Thus, the present data underscore both the need for testing multiple stimulus modalities in procedures used to examine abstract concept learning and the importance of considering the adaptive advantages of particular stimulus discriminations over others. Further, in their review of cross-modal transfer, Ettlinger and Wilson (1990) noted that studying cross-modal performance in a variety of tasks and procedures within and across species could shed light on the neural pathways that may be involved in learning abstract relations. This led us to Experiment 2 that extended the use of 3D stimuli.
Experiment 2
In Experiment 2, we used a different procedure requiring relational learning, a variation of the rodent Odor Span Test (OST) developed by Dudchenko et al. (2000) to study working memory. The OST can be described as an incrementing non-match-to-sample procedure. In the OST, rats learn to respond to session novel olfactory stimuli each day. We have adapted the original procedure so that rats are tested in a large circular arena fitted with 18 equidistant holes in which plastic cups filled with scented sand or covered by odorized lids (e.g., April et al., 2013) are placed. On Trial 1, there is only one cup in the arena. The rat responds by digging or by removing the odorized lid to obtain a reinforcer buried in the sand in the plastic cup. On Trial 2, there are two cups; one has a lid or sand with the same odor as in Trial 1, the other has a session-novel odor. Responses to the cup with the session-novel odor result in reinforcement. Trials in the session continue, always with one session-novel odor and at least one previously presented odor as a comparison. Comparison stimuli are selected from the pool of scents that have been presented already that session. Rats perform with high accuracy on this procedure with memory loads up to 72–100 odors in a single session (April et al., 2013; Bratch et al., 2016) and with two, five, or ten comparison cups on each trial (April et al., 2013).
In the original study by Dudchenko et al. (2000), the researchers were interested in measuring memory span, or the number of correct trials before the first incorrect choice is made. Dudchenko et al. (2000) found that rats had an average memory span just under 8, thus the task appeared to translate to human working memory. Further, researchers compared this non-spatial memory task to an incrementing spatial span version of the task. In the spatial span task, rats received reinforcement for responding to non-odorized cups in new locations in the arena. They found that rats performed accurately on both tasks, but that span was lower in the spatial task. Then, they investigated whether hippocampal lesions disrupted performance on either task. Hippocampal lesions disrupted spatial span performance, as expected, but did not disrupt OST performance.
As noted above, if percent correct in the session is featured as the dependent variable rather than span, rats appear not to show the limited capacity of number of stimuli to remember that was originally reported by Dudchenko et al. (2000). Still, the OST is considered the “gold standard” in terms of rodent models of working memory impairment in schizophrenia (Dudchenko et al., 2013).
Thus, the purpose of Experiment 2 was twofold. First, we wanted to see whether rats could learn the incrementing non-match to sample procedure with three-dimensional stimuli similar to those used in Experiment 1. Second, if so, this might produce a more challenging non-spatial version of a span task that could be more useful in working memory research.
Method
Subjects
Four male Sprague Dawley rats (F15, G2, G14, and H14) began training at approximately 6 months of age. Housing and testing conditions were like those described in Experiment 1.
Apparatus
Testing took place on a circular tabletop arena (29.2 cm high and 94 cm in diameter) enclosed with sheet metal baffling (32 cm high). The table floor had a total of 18 circular holes 5.5 cm in diameter, six equidistant in the center, and 12 equidistant in the outer ring (see Fig. 7). During each trial, 2-oz plastic cups were placed in each hole, each filled halfway with white play sand. A white noise generator was used to reduce distractions during testing.
Experiment 2 stimuli and apparatus. Note. Stimuli used for simple discrimination are shown on row 2 of the group of 30 object stimuli. Object 8 was the S- and Object 10 was the S+
Stimuli
Five replicas of 30 different 3D shapes were constructed from LegoTM pieces (see Fig. 7). Objects were designed to be visually distinctive from each other in both size and color. Shapes were glued onto plastic lids that fit over the 2-oz stimulus cups. 3D shapes were uniformly scented by storing them on wire racks in containers with pecan oil (Great American Spice Company) saturated paper towels placed below the racks.
Procedure
Habituation and initial training
Rats were habituated to the arena by being allowed to explore freely and retrieve sucrose pellets from each cup in the arena during 5-min sessions. Once rats were reliably retrieving pellets, plain lids without shapes were introduced. At first the lids were placed next to the cups and rats were allowed to retrieve sucrose pellets. Then the lids were placed covering 10% of the cup until the rat was reliably retrieving sucrose pellets. Then the lid was moved to cover 50%, then 75%, then 90% of the cup until the rat was readily removing the lids at 100% coverage. Next, lids with shapes were introduced. Rats were given 12 trials, each with a different shape, and had to remove the 3D lid to retrieve the sucrose pellet.
Incrementing non-match to sample training
Once rats were removing the 3D lids with Legos, they started the incrementing non-match to sample (INMTS) training. Each session consisted of twelve trials. On Trial 1, there was only one 3D stimulus; removal of the lid resulted in reinforcement and the rat was removed from the arena and placed in a holding cage for a 15-s inter-trial interval. On Trial 2, two stimuli, a replica of the 3D shape from Trial 1 (S-) and a session-novel 3D shape (S+), were placed in the arena in randomly determined locations. Removal of the lid with the novel 3D object was reinforced. Trials continued in this way with a session-novel shape and a replica of a previously presented shape on each trial. The S- on each trial was selected randomly from the previously presented shapes.
A correction procedure was in place such that if the rat responded to a previously presented shape (S-), he was allowed to continue until he removed the correct lid and obtained the sucrose pellet. During this initial training, if a mistake was made, the next trial consisted of only one session-novel 3D lid, followed by incrementing trials. Once a mistake was made, none of the previously presented shapes were used again that day. Once rats completed at least five correct trials in a row for at least three consecutive days, they advanced to the 3D Span Task.
3D span task
The 3D Span Task (3DST) was similar to the INMTS task except that the trials did not “reset” after incorrect trials. If the rat made an incorrect response, he was allowed to navigate to the S+ and collect the reinforcer, then the next trial would still have two comparisons. While learning the 3DST, rats were tested on two daily sessions of seven trials each (memory load of seven). Stimuli used in the first session were not used in the second session.
Once rats were performing at 85% or higher for three consecutive days on seven-trial sessions, they were advanced to 3D Span 9, then 11, then 13, and so on, to determine accuracy at a variety of memory loads. Because their performance was expected to decline across memory loads, we used a stability criterion rather than a mastery criterion to advance to more sessions with more stimuli. Stability was defined as accuracy that was consistent for 6 days, with the difference between the average percent correct of the first 3 days and the last 3 days not being more than 10% of the grand mean of the 6 days. Percent correct, span (number of correct trials before an error was made minus one), and longest run scores for the different memory load levels were analyzed. Longest run was defined as the number of consecutive correct responses at any time during the session.
To control for the rat’s being able to smell the sucrose pellet under the S+ lid, “no bait” trials were conducted for about half of the testing sessions during 3D Span 9 for Subjects F15 and G2. For these trials, a reinforcer was dropped into the cup by the experimenter using tweezers after selection of the S+. Rats were able to perform accurately on the no bait trials and there was no significant difference in percent correct between no bait trials (M = 78.29, SD = .27) and baited trials (M = 73.17, SD = .15), t(40) = 1.31, p =.196.
Results and discussion
Two of the subjects (F15 and G2) learned the 3D span task. However, the other two rats (G14 and H14) did not meet mastery criteria on the initial resetting incrementing non-match-to-sample training procedure even after 41–55 sessions of testing. Accuracies for G14 and H14 were consistently below 60% and these animals were not tested further.
As Subject F15 was the first rat to be tested on the 3D Span Task, his training history reflected several modifications to the steps outlined in the Method section above. Upon habituation to the arena and shaping of lid removal, F15 began the resetting, incrementing non-match to sample training with 12 trials (12 different objects) in each session (see Fig. 8). However, over 23 days of testing, his performance was quite variable and only averaged 48.7% correct. Thus, we implemented a simple discrimination phase using two shapes to make sure the rat could distinguish them. On these simple discrimination trials, object numbers 10 and 8 were designated as S+ and S-, respectively (see Fig. 7). Subject F15 was tested for 20 sessions of simple discrimination and attained 95% accuracy on the last five sessions as seen in Fig. 7. These two shapes were never used again in the 3DST for F15.
Thus, F15 was moved back to INMTS training with 12 trials per session. Still his performance was below 80% correct; sessions were changed to two seven-trial sessions a day, with performance averaged across the two sessions each day (see Fig. 8, 3D Span 7). On the last 5 days of this phase, he averaged 90% correct and advanced to 3D Span 9. In this phase, F15 was tested for only one session a day with nine trials in each session. He was tested a total of 16 days in this phase, with an average of 79.4% correct, but testing was then discontinued as the laboratory was moved to another building.
Subject G2 met mastery criterion in the resetting INMTS training in 4 days. He was moved immediately to a once a day, 3D Span 7 procedure as seen in Fig. 9. After 16 days, G2 was performing at 85% correct for three consecutive days and advanced to 3D Span 9. In this phase, G2’s percent correct responding stabilized after 13 sessions, averaging 90.8% correct in the last six sessions. He maintained high levels of accuracy with transitions to 3D Spans 11 and 13 (97% and 92.3% correct, respectively). Performance was stable but dropped somewhat at 3D Spans 15 and 17 (83.3% and 86%, respectively).
Within session performance is shown in Fig. 10 for the last 6 days of the highest span procedure each rat received (top panel: F15 at 3D Span 9; bottom panel: G2 at 3D Span 17). Performance is presented in blocks of two trials once two comparison stimuli were in place. Both rats maintained above chance performance across the session, however, their accuracy patterns differed. F15 showed a shallow decrease in accuracy as the memory load increased across the session but it is important to note that this rat had not reached stability or mastery criteria at the point testing was halted. In contrast, G2 maintained above 80% accuracy on most trial blocks within the session even with memory loads up to 17 and showed 100% accuracy on the last two trial blocks of all sessions. Clearly, his performance did not deteriorate across the session. These findings were generally consistent with within-session analyses of OST performances which typically show only a slight decrease in accuracy at comparable memory loads (e.g., April et al., 2013; Galizio et al., 2013; MacQueen et al., 2011).
Table 3 shows span and longest run for F15 and G2. Longest run was consistently higher than span in both animals which is consistent with findings from the OST (April et al. 2013; Galizio et al., 2013, 2016). Span was also more variable demonstrating that early errors do not necessarily indicate a limit in memory capacity. Again, these patterns were noted in the OST studies cited above.
A direct comparison of INMTS with 3D versus odor stimuli was not conducted in the present study, but it is clear that the 3DST was more difficult for rats to learn than the traditional OST. Using similar training procedures, virtually all rats acquire accurate OST performances with 24 or more stimuli in ten–20 sessions (e.g., April et al., 2013; Galizio et al., 2013; MacQueen et al., 2011). In contrast, two of the four rats trained with 3D stimuli never acquired the span task. Subject F15 also performed poorly in the initial INMTS training, but subsequently overall percent correct for both F15 and G2 showed that rats can acquire an INMTS task with 3D stimuli at accuracies comparable to the OST. Further, spans and longest runs were comparable to those observed in the OST (April et al., 2013). In addition, the use of 3D objects that were uniformly scented (plastic with pecan oil) ruled out odor cues as a basis for discrimination in this task.
General discussion
Two types of relational learning were studied with 3D objects in these experiments. In Experiment 1, we found that rats showed generalized non-matching relational learning with two different training modalities (olfactory and 3D). Further, we found that there was evidence of immediate cross-modal transfer of the relation from 3D to olfactory but not from olfactory to 3D stimuli. We noted that the use of 3D objects as training stimuli resulted in a less successful and slower acquisition of the original non-matching task compared to acquisitioacquisition of the original non- Similarly, in Experiment 2, two of four rats were able to acquire an incrementing non-match-to-sample task with 3D stimuli (3D Span Task), but more training was required in those that did acquire the task compared to what is typically required in the Odor Span Task. Still, final accuracies with the 3D stimuli were comparable to those with the olfactory stimuli.
The findings of Experiment 1 replicated previous reports of generalized NMTS by rats after training with olfactory stimuli (April et al., 2011; Bruce et al., 2018; Lazarowski et al., 2019) as evidenced by accuracies with novel stimuli in the same modality that were comparable to performances on trained relations (cf. Katz & Wright, 2006). Further, rats learned NMTS with 3D stimuli and generalized to novel stimuli, thus extending the research of Mumby et al. (1990) and Rothblat and Hayes (1987), who used trial unique but not novel stimuli in their procedures. While both Mumby et al. (1990) and Rothblat and Hayes (1987) used duplicates of their stimuli to eliminate scent marking as an explanation for performance, their junk objects were constructed from different materials (wood, plastic, and metal). Thus, while it seemed that their non-matching task involved a visual discrimination, the animals may actually have been responding based on discrimination of olfactory cues associated with the different stimulus materials. This potential source of stimulus control was eliminated in the current study as all objects were composed of the same materials.
While there was no immediate transfer from olfactory to 3D stimuli in the cross-modality probe transfer tests, the one subject that learned the 3D task first did show cross-modal transfer from 3D to olfactory stimuli. This rat was 90% accurate on the first set of cross-modality probe trials, similar to accuracy on baseline trained relations. This finding supports previous reports of cross-modal transfer of the same/different or identity/oddity relation in two marine mammals (Herman et al., 1989; Scholtyssek et al., 2013) and capuchin monkeys (Truppa et al., 2010), but is the first to be reported in rats. It is curious that transfer from 3D stimuli to odors was successful, but from odors to 3D stimuli was not. One possible explanation for this is that odor cues are simply more salient than 3D cues for rats. This might make stimulus relations between odors more salient as well. In any case, as noted above, rats do seem to learn a variety of tasks more readily with olfactory stimuli perhaps due to phylogenetic adaptations (Nigrosh et al. 1975; Slotnick, 2001). Consistent with this interpretation is that rats did acquire the non-matching task more readily when the stimuli were olfactory rather than 3D.
An alternative account for the superior performances with olfactory stimuli could be developed that relates to the proximity of the stimulus to the required response. Harrison et al. (1977) observed more rapid learning when the source of the auditory discriminative stimuli was adjacent to the response than when the response was located more distantly. In the present study, the odor source was intrinsic to the plastic lids that rats manipulated to produce reinforcement. The 3D stimuli were located above the lids and thus may not have been as salient. Consistent with such an account, we observed that the topography of lid removal generally involved direct contact between the rat’s paw and the plastic lid with both olfactory and 3D stimuli. Rats rarely made physical contact with the 3D stimuli which could have reduced their salience. That said, Nigrosh et al. (1975) found discrimination learning to be more rapid with olfactory stimuli than with visual or auditory stimuli without obvious differences in proximity between the stimuli and responses. Thus, for rats, it may simply be that the high salience of odor stimuli facilitates relational learning.
The same point might be made regarding the 3D Span Task. Although two rats did acquire the task, two other rats failed to learn the task. In studies of incrementing NMTS with olfactory stimuli (the OST), acquisition is generally quite rapid and virtually all rats trained acquire the task (April et al., 2013; Galizio et al., 2013; MacQueen et al., 2011). Although only two rats acquired the 3D span task, once learned, their performances were quite accurate and showed little evidence of degradation within the session as the memory load increased. These findings were quite similar to the high memory capacities shown in the OST (e.g., April et al., 2013)
Thus, the present two experiments show that complex relational learning in rats is possible with 3D stimuli. Of particular importance, Experiment 1 showed evidence of cross-modal transfer of NMTS. Even though this effect was observed in only one rat, it strengthens support for the argument for abstract concept learning. The present procedures offer a foundation for further investigation of relational learning across multiple modalities.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
April, L. B., Bruce, K., & Galizio, M. (2011). Matching- and nonmatching-to-sample concept learning in rats using olfactory stimuli. Journal of the Experimental Analysis of Behavior, 96, 139–154. https://doi.org/10.1901/jeab.2011.96-139
April, L. B., Bruce, K., & Galizio, M. (2013). The magic number 70 (plus or minus 20): Variables determining performance in the rodent odor span task. Learning and Motivation, 44(3), 143–158. https://doi.org/10.1016/j.lmot.2013.03.001
Berryman, R., Cumming, W. W., Cohen, L. R., & Johnson, D. F. (1965). Acquisition and transfer of simultaneous oddity. Psychological Reports, 17, 767–775. https://doi.org/10.2466/pr0.1965.17.3.767
Bodily, K. D., Katz, J. S., & Wright, A. A. (2008). Matching-to-sample abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 34, 178–184. https://doi.org/10.1037/0097-7403.34.1.178
Boysen, S. T. (1996). Individual differences in the cognitive abilities of chimpanzees. In R. W. Wrangham, W. C. McGrew, F. B. M. de Waal, & P. G. Heltne (Eds.), Chimpanzee cultures (pp. 335–346). Harvard University Press.
Boysen, S. T., Berntson, G. G., Hannan, M. B., & Cacioppo, J. T. (1996). Quantity-based interference and symbolic representations in chimpanzees (pan troglodytes). Journal of Experimental Psychology: Animal Behavior Processes, 22(1), 76–86. https://doi.org/10.1037/0097-7403.22.1.76
Bratch, A., Kann, S., Cain, J. A., Wu, J. E., Rivera-Reyes, N., Dalecki, S., Arman, S., Dunn, A., Cooper, S., Corbin, H., Doyle, A. R., Pizzo, M. J., Smith, A. E., & Crystal, J. D. (2016). Working memory systems in the rat. Current Biology, 26(3), 351–355. https://doi.org/10.1016/j.cub.2015.11.068
Brino, A. L. F., Galvão, O. F., Picanço, C. R. F., Barros, R. S., Souza, C. B. A., Goulart, P. R. K., & McIlvane, W. J. (2014). Generalized identity matching-to-sample after multiple-exemplar training in capuchin monkeys. Psychological Record, 64, 693–702. https://doi.org/10.1007/s40732-014-0035-x
Brown, M. F., & Austin, B. P. (2021). Bees and abstract concepts. Current Opinion in Behavioral Sciences, 37, 140–145. https://doi.org/10.1016/j.cobeha.2020.12.002
Bruce, K., Dyer, K., Mathews, M., Nealley, C., Phasukkan, T., Prichard, A., & Galizio, M. (2018). Successive odor matching-and non-matching-to-sample in rats: A reversal design. Behavioural Processes, 155, 26–32. https://doi.org/10.1016/j.beproc.2017.07.003
Carter, D. E., & Werner, T. J. (1978). Complex learning and information processing by pigeons: A critical analysis. Journal of the Experimental Analysis of Behavior, 29(3), 565–601. https://doi.org/10.1901/jeab.1978.29-565
D'amato, M. R., Salmon, D. P., & Colombo, M. (1985). Extent and limits of the matching concept in monkeys (Cebus apella). Journal of Experimental Psychology: Animal Behavior Processes, 11(1), 35. https://doi.org/10.1037/0097-7403.11.1.35
Daniel, T. A., Wright, A. A., & Katz, J. S. (2015). Abstract-concept learning of difference in pigeons. Animal Cognition, 18, 831–837. https://doi.org/10.1037/h0035970
Denny, M., Clos, C., & Rilling, M. (1989). Delayed matching-to-sample in rats in a Y-maze: Instances of facilitation and immediate cross-modal transfer. Bulletin of the Psychonomic Society, 27(2), 141–144. https://doi.org/10.3758/BF03329923
Dube, W. V., Callahan, T. D., & McIlvane, W. J. (1993). Serial reversals of concurrent auditory discrimination in rats. The Psychological Record, 43(3), 429–440.
Dudchenko, P. A., Talpos, J., Young, J., & Baxter, M. G. (2013). Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neuroscience & Biobehavioral Reviews, 37(9), 2111–2124. https://doi.org/10.1016/j.neubiorev.2012.03.003
Dudchenko, P. A., Wood, E. R., & Eichenbaum, H. (2000). Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. Journal of Neuroscience, 20(8), 2964–2977. https://doi.org/10.1523/JNEUROSCI.20-08-02964.2000
Ettlinger, G., & Wilson, W. A. (1990). Cross-modal performance: Behavioural processes, phylogenetic considerations and neural mechanisms. Behavioural Brain Research, 40(3), 169–192. https://doi.org/10.1016/0166-4328(90)90075-P
Galizio, M., April, B., Deal, M., Hawkey, A., Panoz-Brown, D., Prichard, A., & Bruce, K. (2016). Behavioral pharmacology of the odor span task: Effects of flunitrazepam, ketamine, methamphetamine and methylphenidate. Journal of the Experimental Analysis of Behavior, 106(3), 173–194. https://doi.org/10.1002/jeab.224
Galizio, M., Deal, M., Hawkey, A., & April, B. (2013). Working memory in the odor span task: Effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology, 225(2), 397–406. https://doi.org/10.1007/s00213-012-2825-7
Galizio, M., Mathews, M., Prichard, A., & Bruce, K. E. (2018). Generalized identity in a successive matching-to-sample procedure in rats: Effects of number of exemplars and a masking stimulus. Journal of the Experimental Analysis of Behavior, 110(3), 366–379. https://doi.org/10.1002/jeab.483
Giurfa, M., Zhang, S., & Jenett, A. (2001). The concepts of 'sameness' and 'difference' in an insect. Nature, 410, 930–933. https://doi.org/10.1038/35073582
Harrison, J. M., Iversen, S. D., & Pratt, S. R. (1977). Control of responding by location of auditory stimuli: Adjacency of sound and response. Journal of the Experimental Analysis of Behavior, 28(3), 243–251. https://doi.org/10.1901/jeab.1977.28-243
Herman, L. M., Hovancik, J. R., Gory, J. D., & Bradshaw, G. L. (1989). Generalization of visual matching by a bottle nosed dolphin (Tursiops truncates): Evidence for invariance of cognitive performance with visual and auditory materials. Journal of Experimental Psychology: Animal Behavior Processes, 15, 124–136. https://doi.org/10.1037/0097-7403.15.2.124
Iversen, I. (1993). Acquisition of matching-to-sample performance in rats using visual stimuli on nose keys. Journal of the Experimental Analysis of Behavior, 59, 471–482. https://doi.org/10.1901/jeab.1993.59-471
Iversen, I. (1997). Matching-to-sample performance in rats: A case of mistaken identity? Journal of the Experimental Analysis of Behavior, 68, 27–45. https://doi.org/10.1901/jeab.1997.68-27
Kastak, D., & Schusterman, R. (1994). Transfer of visual identity matching-to-sample in two California Sea lions (Zalophus californianus). Animal Learning & Behavior, 22, 427–435. https://doi.org/10.3758/BF03209162
Katz, J. S., & Wright, A. A. (2006). Mechanisms of same/different abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 32, 80–86. https://doi.org/10.1037/0097-7403.28.4.358
Katz, J. S., Bodily, K. D., & Wright, A. A. (2008). Learning strategies in matching to sample: If-then and configural learning by pigeons. Behavioral Processes, 77, 223–230. https://doi.org/10.1016/j.beproc.2007.10.011
Katz, J. S., Wright, A. A., & Bachevalier, J. (2002). Mechanisms of same/different abstract-concept learning by rhesus monkeys (Macaca mulatta). Journal of Experimental Psychology: Animal Behavior Processes, 28, 358–368. https://doi.org/10.1037/0097-7403.28.4.358
Katz, J. S., Wright, A. A., & Bodily, K. D. (2007). Issues in the comparative cognition of abstract-concept learning. Comparative Cognition & Behavior Reviews, 2, 79–92. https://doi.org/10.3819/ccbr.2008.20005
Keller, F., & Schoenfeld, W. (1950). Generalization and discrimination. Principles of psychology: A systematic text in the science of behavior (pp. 115–163). Appleton Century-Crofts.
Lazareva, O. F., & Wasserman, E. A. (2008). Categories and concepts in animals. In R. Menzel et al. (Eds.), Learning and memory-a comprehensive reference. Vol. II: Behavioral approaches (p. (197226).). Elsevier.
Lazarowski, L., Davila, A., Krichbaum, S., Cox, E., Smith, J. G., Waggoner, L. P., & Katz, J. S. (2021). Matching-to-sample abstract-concept learning by dogs (Canis familiaris). Journal of Experimental Psychology: Animal Learning and Cognition. https://doi.org/10.1037/xan0000281
Lazarowski, L., Goodman, A., Galizio, M., & Bruce, K. (2019). Effects of set size on identity and oddity abstract-concept learning in rats. Animal Cognition, 22(5), 733–742. https://doi.org/10.1007/s10071-019-01270-5
Lu, X. M., Slotnick, B. M., & Silberberg, A. M. (1993). Odor matching and odor memory in the rat. Physiology and Behavior, 53, 795–804. https://doi.org/10.1016/0031-9384(93)90191-H
Mackintosh, N. J. (2000). Abstraction and discrimination. In C. Heyes & L. Huber (Eds.), The evolution of cognition (pp. 123–141). The MIT Press.
MacQueen, D. A., Bullard, L., & Galizio, M. (2011). Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiology of Learning and Memory, 95(1), 57–63. https://doi.org/10.1016/j.nlm.2010.11.004
Magnotti, J. F., Katz, J. S., Wright, A. A., & Kelly, D. M. (2015). Superior abstract-concept learning by Clark's nutcrackers (Nucifraga columbiana). Biology Letters, 11(5), 20150148. https://doi.org/10.1098/rsbl.2015.0148
McIlvane, W. J. (2013). Simple and complex discrimination learning. In G. J. Madden et al. (Eds.), APA handbook of behavior analysis: Translating principles into practice (Vol. 2, pp. 129–163). American Psychological Association.
Mumby, D. G., Pinel, P. J., & Wood, E. R. (1990). Nonrecurring-items delayed non-matching-to-sample in rats: A new paradigm for testing nonspatial working memory. Psychobiology, 18(3), 321–326.
Nakagawa, E. (1993). Relational rule learning in the rat. Psychobiology, 21(4), 293–298.
Nigrosh, B. J., Slotnick, B. M., & Nevin, J. A. (1975). Olfactory discrimination, reversal learning, and stimulus control in rats. Journal of Comparative and Physiological Psychology, 89(4), 285–294. https://doi.org/10.1037/h0076821
Oden, D. L., Thompson, R. K. R., & Premack, D. (1988). Spontaneous transfer of matching by infant chimpanzees (pan troglodytes). Journal of Experimental Psychology: Animal Behavior Processes, 14, 140–145. https://doi.org/10.1037/0097-7403.14.2.140
Pena, T., Pitts, R. C., & Galizio, M. (2006). Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior, 85, 203–221. https://doi.org/10.1901/jeab.2006.111-04
Prichard, A., Panoz-Brown, D., Bruce, K., & Galizio, M. (2015). Emergent identity but not symmetry following successive olfactory discrimination training in rats. Journal of the Experimental Analysis of Behavior, 104(2), 133–145. https://doi.org/10.1002/jeab.169
Rothblat, L. A., & Hayes, L. L. (1987). Short-term object recognition memory in the rat: Non-matching with trial-unique junk stimuli. Behavioral Neuroscience, 101, 587–590. https://doi.org/10.1037/0735-7044.101.4.587
Russell, F., & Burke, D. (2016). Conditional same/different concept learning in the short-beaked echidna (Tachyglossus aculeatus). Journal of the Experimental Analysis of Behavior, 105, 133–154. https://doi.org/10.1002/jeab.185
Scholtyssek, C., Kelber, A., Hanke, F. D., & Dehnhardt, G. (2013). A harbor seal can transfer the same/different concept to new stimulus dimensions. Animal Cognition, 16, 915–925. https://doi.org/10.1007/s10071-013-0624-0
Slotnick, B. (2001). Animal cognition and the rat olfactory system. Trends in Cognitive Sciences, 5(5), 216–222. https://doi.org/10.1016/S1364-6613(00)01625-9
Taniuchi, T., Miyazaki, R., & Siddik, M. A. B. (2017). Concurrent learning of multiple oddity discrimination in rats. Behavioural Processes, 140, 6–15. https://doi.org/10.1016/j.beproc.2017.03.008
Thomas, R. K., & Noble, L. M. (1988). Visual and olfactory learning in rats: What evidence is necessary to show conceptual behavior? Animal Learning and Behavior, 16, 157–163. https://doi.org/10.3758/BF03209059
Thompson, R. K. R., Oden, D. L., & Boysen, S. T. (1997). Language-naive chimpanzees (pan troglodytes) judge relations between relations in a conceptual matching-to-sample task. Journal of Experimental Psychology: Animal Behavior Processes, 23, 31–43.
Tran, T. D., Adducci, L. C., DeBartolo, L. D., Bower, B. A., & Delay, E. R. (1994). Transfer of visual and haptic maze learning in rats. Animal Learning & Behavior, 22(4), 421–426. https://doi.org/10.3758/BF03209161
Truppa, V., Garofoli, D., Castorina, G., Mortari, E. P., Natale, F., & Visalberghi, E. (2010). Identity concept learning in matching-to-sample tasks by tufted capuchin monkeys (Cebus apella). Animal Cognition, 13, 835–848. https://doi.org/10.1007/s10071-010-0332-y
Vonk, J. (2003). Gorilla (Gorilla gorilla gorilla) and orangutan (pongo abelii) understanding of first-and second-order relations. Animal Cognition, 6, 77–86. https://doi.org/10.1007/s10071-003-0159-x
Wilson, B., Mackintosh, N. J., & Boakes, R. A. (1985). Transfer of relational rules in matching and oddity learning in pigeons and corvids. Quarterly Journal of Experimental Psychology, 37B, 313–332. https://doi.org/10.1080/14640748508401173
Wright, A. A., Cook, R. G., Rivera, J. J., Sands, S. F., & Delius, J. D. (1988). Concept learning by pigeons: Matching to sample with trial-unique video picture stimuli. Animal Learning and Behavior, 16, 436–444. https://doi.org/10.3758/BF03209384
Wright, A. A., Magnotti, J. F., Katz, J. S., Leonard, K., & Kelly, D. M. (2016). Concept learning set-size functions for Clark's nutcrackers. Journal of the Experimental Analysis of Behavior, 105, 76–84. https://doi.org/10.1002/jeab.174
Wright, A. A., & Katz, J. S. (2007). Generalization hypothesis of abstract-concept learning: Learning strategies and related issues in Macaca mulatta, Cebus paella, and Columbia livia. Journal of Comparative Psychology, 121(4), 387–397. https://doi.org/10.1037/0735-7036.121.4.387
Wright, A. A., Rivera, J. J., Katz, J. S., & Bachevalier, J. (2003). Abstract-concept learning and list memory processing by capuchin and rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes, 29, 184–198. https://doi.org/10.1037/0097-7403.29.3.184
Acknowledgements
The authors thank Emily Berkey, Carrie Branch, Mallory Gleason, Adam Goodman, Alyse Kaszubski, Ally Mack, and Alex McLean for assistance in data collection. Funding was provided by grants from the UNC Wilmington CSURF program.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to design, analysis, and writing. RE and VO collected data.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare no conflicts of interest or competing interests.
Ethics approval
All procedures were reviewed and approved by the UNCW Institutional Animal Care and Use Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Open practices statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruce, K., Eure, R., O’Connor, V. et al. Generalized, cross-modal, and incrementing non-matching-to-sample in rats. Learn Behav 51, 88–107 (2023). https://doi.org/10.3758/s13420-023-00571-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-023-00571-7