Abstract
Social learning during foraging has been found in a wide range of animals, including numerous bird species. Still, the mechanisms underlying this cognitive capacity remain largely unstudied and the use of divergent methods limits our understanding of their taxonomic distribution. Using an ecologically relevant design, the open diffusion experiment, we tested whether 11 Southern ground-hornbills (Bucorvus leadbeateri) were able to show imitation on the two-action task. Three experimental groups were created. In the slide and pull group, subjects (‘observers’) watched a trained conspecific (‘demonstrator’) opening a box using a specific technique. Naïve individuals from the control group, however, did not receive a social demonstration. All birds of the slide and pull group succeeded in opening the box, whereas all subjects of the control group failed the task. We found consistent inter-individual differences among some observers, with only two birds (one in each group) using the same technique and part of the box contacted by the demonstrator. Our results suggest that at least fine-tune enhancement underlies behavioural diffusion in this novel model species, which provides new research opportunities with direct implications for conservation.
Similar content being viewed by others
Introduction
Social learning, which, in its broad sense, involves the capacity to acquire information from other individuals (Aplin et al., 2015), is taxonomically widespread and occurs regardless of different aspects of the species’ social organization (e.g., solitary (Guttridge et al., 2013) and group-living species (van Leeuwen et al., 2014)), ecology (e.g., generalist and specialist species (Goulson et al., 2013)), or life-history traits (e.g., relatively ‘small-brained’ (Benson-Amram et al., 2014), and ‘large-brained’ species (Sargeant & Mann, 2009)). To our knowledge, all animals experimentally tested so far have been able to exploit the knowledge of conspecifics, either directly or after further investigations (but see Farrar et al., 2021, for evidence of publication bias in animal cognition research).
Birds are particularly prone to learn socially (Slagsvold & Wiebe, 2011), as demonstrated by several captive and field studies in multiple biological contexts of adaptive significance (e.g., Brown & Fawcett, 2005; Griffin, 2004; Lefebvre & Bouchard, 2003). In the extensively studied foraging context, for instance, a wide range of avian families has been capable of learning a novel feeding technique by observing a conspecific demonstrating the behaviour (e.g., Anatidae: Fritz et al., 2000; Columbidae: Bouchard et al., 2007; Corvidae: Fritz & Kotrschal, 1999; Falconidae: Biondi et al., 2010; Paridae: Aplin et al., 2013; Pelecanidae: Danel et al., 2020; Ploceidae: Danel et al., 2018; Stercorariidae: Danel et al., 2019).
Social influence on foraging behaviour takes many forms, ranging from simple to more complex cognitive processes (e.g., Canteloup et al., 2020; Custance et al., 2006; Whiten et al., 1999; for a review, see Kendal et al., 2018). In this study, we refer to the definitions provided by Rørvang et al. (2018) to distinguish between those mechanisms. On one hand, social transmission includes mere social influence on behavioural acquisition (e.g., social facilitation and learning enhancement: Thorpe, 1963; Spence, 1937; Zajonc, 1965). This can be illustrated by a bird approaching a specific area due to the presence of other conspecifics foraging at this location (local enhancement, e.g., Picard et al., 2017). Low-level social learning mechanisms seem to prevail in birds, as they are often sufficient to transfer information reliably (Nicol, 1995; Slagsvold & Wiebe, 2011). True social learning (emulation and imitation), on the other hand, is thought to require more complex cognitive abilities (Rørvang et al., 2018). For instance, depressing a lever using the same topography response as a conspecific demonstrator (beak or foot) indicates imitation learning in Japanese quails (Coturnix japonica: Akins & Zentall, 1996).
One of the most common, well-established paradigms used to demonstrate high-level social learning mechanisms in non-human animals is the two-action procedure (Dawson & Foss, 1965). Observers are exposed to one of two (or more, e.g., Dawson & Foss, 1965) demonstrated actions (‘response topographies’) to obtain a reward in an apparatus (Zentall, 2004). In this paradigm, or similar approaches and methodological variations, the demonstrated techniques can involve (i) distinct environmental outcomes and topographies (e.g., stopper pushed in/pulled out of the device: Campbell et al., 1999), (ii) distinct environmental outcomes depending on the directional movements of a same topography (bidirectional control, e.g., pushing on the left or right side: Heyes & Dawson, 1990), or (iii) the same environmental outcome with different topographies (e.g., stepping on or pecking at the treadle: Akins & Zentall, 1998). Emulation or imitation processes are assumed to be involved if observers reproduce the demonstrator’s action above the levels expected by chance (Picard et al., 2017).
Originally, the two-action procedure has been administered within a dyadic setting: one observer is allowed to watch a single physically separated trained individual (Coussi-Korbel & Fragaszy, 1995). Following the social demonstration, the observer is isolated and is allowed full access to the apparatus. Observer birds such as budgerigars (Dawson & Foss, 1965; but see Galef et al., 1986), starlings (Campbell et al., 1999; Fawcett et al., 2002), pigeons (Kaiser et al., 1997; Zentall et al., 1996), or quails (Akins & Zentall, 1998; Zentall et al., 1996) have been reported to emulate or potentially imitate the demonstrated method with this procedure.
However, although the two-action task based on dyadic tests has allowed rigorous differentiation between individual and social learning, as well as analyses of the learning process (Dindo et al., 2008; Whiten & Mesoudi, 2008; Zentall, 2004), this setting is not ecologically relevant for socially foraging species (Reader & Biro, 2010; Whiten & Mesoudi, 2008). Under natural conditions, the opportunity for one group member to observe another experienced individual in total isolation is highly unlikely. Several social factors, resulting from the interactions among group members, come into play and influence the acquisition of a novel foraging behaviour (Aplin et al., 2012; Coussi-Korbel & Fragaszy, 1995). For instance, the social learning performance of subordinate individuals may not emerge first hand when dominant models monopolize feeding resources (Boinski, 1999; Kappeler, 1987; Picard et al., 2017; van de Waal & Whiten, 2012). Moreover, individual propensity to show social learning may vary depending on whether aggression or social tolerance is exhibited within the group (e.g., Cadieu et al., 2010). Novel techniques, such as scrounging (pilfering), may also be displayed. Scroungers can obtain the reward without using social learning per se (e.g., Schnöll, 2010), which may inhibit or facilitate the appearance of this cognitive capacity later on (Giraldeau & Lefebvre, 1987; Inoue-Nakamura & Matsuzawa, 1997).
One experimental way to investigate social learning mechanisms in ecologically valid group contexts is afforded by the open diffusion experiment (Whiten & Mesoudi, 2008). Typically, two experimental groups are selected and an individual within each group is trained to use one of two alternative foraging methods (e.g., Claidiere et al., 2013; Dindo et al., 2008). All group members are then allowed to watch simultaneously the technique displayed by the trained subject, thus mirroring natural social situations. In birds, only two species have been tested with this method using the two-action paradigm: Amazon parrots (Amazona amazonica: Picard et al., 2017) and blue tits (Cyanistes caeruleus: Aplin et al., 2013, 2015). From those studied species, only the latter has demonstrated the ability to favour the initially demonstrated foraging technique within wild sub-populations (Aplin et al., 2015).
Besides the previously cited highly controlled aspect of the dyadic testing, along with the difficulty of the experimental design applied (Picard et al., 2017; Tennie et al., 2006), imitating another’s foraging behaviour has been assumed to rely on ecological factors such as the paucity or complexity of the food resource (Zentall, 2004). For instance, high-fidelity social learning mechanisms may be needed to know how to process food items, such as handling certain prey types (Thornton & Clutton-Brock, 2011). However, we still know little about the influence of complex foraging on social learning mechanisms in birds and, more generally, on the processes involved in the acquisition of novel foraging behaviours.
To address these gaps, it is necessary to expand the administration of the two-action task based on open diffusion experiments in socially foraging species. A truly comparative view would also provide an understanding of the mechanisms underlying the acquisition and evolution of cultural traditions in animals (Picard et al., 2017), including humans (Galef, 1992; Laland & Hoppitt, 2003). Noteworthy, investigating social cognition in endangered species can give rise to substantial conservation implications (Greggor et al., 2014, 2017; Greggor, Price, & Shier, 2019b). For instance, successful reintroductions of avian species have been aided by tapping into their social learning abilities (McLean et al., 1999; Urbanek et al., 2010). This conservation-cognition interface needs to be taken into consideration when studying learning processes in threatened or endangered systems (Greggor et al., 2019a)
In this context, the Southern ground-hornbill (Bucorvus leadbeateri) makes an excellent biological model for investigating social learning mechanisms during foraging. This bird, listed as Vulnerable globally and Endangered in South Africa (Taylor & Kemp, 2015), is endemic to Africa south of the equator where it frequents open habitats such as savannahs, grasslands, or open woodlands (Kemp & Boesman, 2019). Ground-hornbills are faunivorous, feeding on a wide range of small animals and hunting in groups when the prey is larger. They are also opportunistic scavengers and have been reported to feed on fruits and seeds on occasion (Kemp & Boesman, 2019).
This species is a monogamous cooperative breeder: the complex social unit, which ranges in size from two to 12 individuals, consists of a dominant pair (alpha male and female) assisted by adult and immature male helpers (Kemp & Boesman, 2019). Each group resides permanently in extensive territories (between 50 and 100 km2) that are fiercely defended (Kemp & Kemp, 1980; Wyness, 2011; Zoghby et al., 2015). Although ground-hornbills are aggressive and territorial, they are highly tolerant towards group members (Kemp & Kemp, 1980), and thus have ample opportunity to use social information (Coussi-Korbel & Fragaszy, 1995). The juvenile’s prolonged period of nutritional dependence on the group members (up to 2 years) and high parental investment (3–5 years) enable the young to learn a great deal socially about their physical and social environment (Knight, 1990). Such rare and prolonged parental provisioning has been suggested to correlate with the time needed by juvenile birds to learn complex skills, such as intricate foraging techniques (Hunt et al., 2012). For instance, in ground-hornbills social learning might be highly valuable during the acquisition of specific feeding methods, for instance snake-handling or tortoise-excavation skills, which would otherwise be highly hazardous or difficult to develop through exploratory asocial learning. Replicating these complex social habits, by placing young captive individuals into ‘bush schools’, where they learn from an experienced conspecific (usually the alpha male), is vital to promote successful reintroductions in the wild (Kemp et al., 2020). These features, combined with complex social interactions (Kemp & Kemp, 1980), specific ecological traits (e.g., group foraging, opportunism), and other life histories (e.g., large relative brain size; unusually long lifespan: 70 years; low productivity: maximum one fledged per group per year; late maturity: more than 10 years), are believed to facilitate the emergence of social learning in animals (Coussi-Korbel & Fragaszy, 1995; Gajdon et al., 2004; Gariépy et al., 2014; Greco et al., 2013; Lefebvre, 2000; Lefebvre et al., 1997; Ricklefs, 2004). So far, however, only one experiment has tested this species’ cognitive ability to solve problems in the physical domain (Danel et al., 2019). In this study, ground-hornbills were able to solve the string-pulling task in five experimental conditions using different string patterns. For instance, some individuals were able to show goal-directed behaviour (two strings are presented but one string is attached to a food reward), to understand that the string has to be physically connected to the reward (two strings are rewarded but only one string is attached to a food reward), and to not rely on perceptual feedback (two rewarded strings are coiled so the strings do not come directly closer when pulled). Social cognition in ground-hornbills, and in the order Bucerotiformes more generally, is poorly understood. Yet, Southern ground-hornbills live in cooperative groups where members exhibit sophisticated behaviours in various biological contexts (Kemp et al., 2020; Knight, 1990). Therefore, this species offers the opportunity for assessing what and how information is transmitted within social foraging groups.
In this study, we aimed at testing the social learning capacity of ground-hornbills using a three-group, two-action paradigm with an open-diffusion approach. Subjects from two treatment groups were exposed to a trained demonstrator, which either (i) slid to the right side or (ii) pulled from top to bottom, the central door of a foraging apparatus containing a food reward. The third group, however, served as a control for asocial learning (individuals did not have the opportunity to watch a demonstrator). For comparative purposes, we built a replica of the two-way foraging box used in Picard et al. (2017), with opening techniques (push/pull) similar to those used in other avian studies (e.g., Dawson & Foss, 1965; Fawcett et al., 2002; Galef Jr. et al., 1986). Given the seeming ubiquity of social transmission mechanisms among birds, we expected learning enhancement to occur in the Southern ground-hornbill (Prediction 1). More specifically, due to the presence of the demonstrator, the apparatus (stimulus enhancement; Spence, 1937) or its location (local enhancement; Thorpe, 1963) should attract observers (i.e., making them touch the box). We also predicted that observers (and subjects that have received previous box-opening training, i.e., the demonstrators, see below) should be able to open the apparatus (Prediction 2). However, stimulus and local enhancement (box opening by trial-and-error learning i.e., using a distinct opening technique and/or directing actions to the sites of the box not contacted by the demonstrator) may be insufficient to explain the extensive learning phase required for acquiring some complex foraging techniques exhibited in the wild (e.g., hunting skills). Therefore, we expect subjects of this species to show imitation learning (straightforward box opening, using the same opening technique and/or directing actions to the same sites of the box touched by the demonstrator; Prediction 3). Finally, since the open diffusion approach allows social interactions during the acquisition of novel foraging behaviours (Coussi-Korbel & Fragaszy, 1995), we predict reporting agonistic and pilfering (‘scrounging’) behaviours within test groups (Prediction 4), which can influence (facilitating or hindering) the acquisition of novel foraging behaviours.
Methods
Subjects
Eleven captive ground-hornbills, ranging in age from 1 to 28 years, participated in this experiment between September and November 2019. Seven subjects were tested at the Baobab Southern Ground-hornbill Conservation Rearing Centre, Mpumalanga, South Africa (six females: Adonsonia, Kalula, Mud, Nkosi, Red Rocks, WitchyWitch; one male: Raymond; Table 1), and four birds at the National Zoological Gardens, Pretoria, South Africa (three females: Hull, Lindada, Makalali; one male: Chiredzi; Table 1). All subjects were individually identified based on stainless steel rings (South African Bird Ringing Unit's – SAFRING) and/or coloured plastic spiral rings, except for one easily recognizable juvenile (Adonsonia). Three experimental groups were created: the pull group (demonstrator: Kalula; observers: Mud, Nkosi, Red Rocks), the slide group (demonstrator: Hull; observers: Chiredzi, Lindada, Makalali), and the control group (naïve individuals: Adonsonia, Raymond, WitchyWitch). For the pull and slide group, the selection criteria of the demonstrator (Kalula: pull group; Hull: slide group) were similar to Picard et al. (2017), that is high levels of food motivation and social tolerance (social middle rank; Table 1). Each group was housed separately in outside aviaries (pull group: 30m2; slide group: 55m2; control group: 55m2). An indoor trained compartment was built (4m2) for both the pull and the slide groups (Fig. S1; Online Supplementary Material (OSM)). The door of the training compartment (which allowed testing each demonstrator in isolation, see Procedure below) was removed during testing for the slide group (Pretoria zoo; Fig. S1 (OSM)). The enclosures contained natural wood perches and artificial nest boxes, and were regularly provided with cardboard boxes, empty plastic bottles, and large pieces of wood for enrichment purposes (Kemp, 2017). Usually, tests took place within the test area each morning (from 8 to 11 a.m.) and each afternoon (from 1 to 5 p.m.). All birds were brought down to 85% of their weight during the time of the experimental period. A one-at-a-time, highly favoured reward (defrosted pieces of mice or chicken heads/legs) was provided during the apparatus’s reloading. These subjects, and this species more generally, had not been tested in any social learning task previously.
Experimental setup and materials
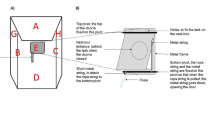
The two-way foraging box consisted of a rectangular black plastic basket (40 cm in length × 30 cm in width × 30 cm in height) fixed to a wood board on its aperture side. A movable platform (15 cm in length, 10 cm in height), with a square handle added to its centre, could be opened by either pulling or sliding (Figs. 1 and 2). The task could be solved only by using the bill (ground-hornbills do not use their feet during feeding: Kemp & Boesman, 2019). While the only way to get the reward successfully with the slide technique was to contact part 5 of the door (Fig. 2), we found that observers showed behavioural variations in the use of the pull technique. Indeed, although the demonstrator was trained with one pull technique (pull part 1; Fig. 2), some subjects also touched other parts of the movable platform to open the box (pull parts 2, 3, and 4; Fig. 2). Thus, a total of five box’s parts (parts 1–4 causing the pull technique, part 5 the slide technique) could be contacted to open the box (Fig. 2). The apparatus was mounted on a portable iron table (70 cm × 30 cm × 20 cm) for the pull group, on two bricks (11 cm in height) for the slide group, and on the ground for the control group. The recording material consisted of two video cameras (Samsung camera HMX-F90, mounted on a tripod; Gopro Hero 5 Black, attached to the fence 110 cm minimum above the ground) that, along with the apparatus, was removed from the test area after each test.
Photograph describing the sites of the door that could be contacted to open the box and reach the food reward. A total of five sites (parts 1–4 causing the pull technique, and part 5 causing the slide technique) was reported: pull part 1 involved inclining the head on the side and inserting the beak in the slot located on the superior part of the door, pull part 2 consisted of grabbing the square located in the centre of the door, pull part 3 involved inserting the beak in the slot located on the right side of the door, pull part 4 was similar to pull part 3 but implied the inferior part of the door, and slide part 5 involved pushing the door on the right to create a sliding movement
Procedure
Habituation and training phase
Test groups (pull group and slide group)
Before the experiment, for 1 h a day over three contiguous days, subjects were habituated to the video cameras located in a compartment within the aviary (Fig. S1). All birds had access to the compartment (the front door of the compartment remained opened during this phase). In the next training phase, the demonstrators were visually isolated from their conspecifics within the compartment (the front door was closed and opaque brown fabric covered each wall), to learn their respective technique (two trials per day, maximum 15 min each). Each demonstrator was trained to retrieve the food reward in the box by (i) pulling its front door (Kalula: pull group) and (ii) sliding its front door on the right side (Hull: slide group). We proceeded as follows: initially, the food reward was available in the opened box. Then, the reward was partly reachable inside the apparatus (we lifted/slid the front door progressively), so while trying to take the reward demonstrators unintentionally pulled/slid the front door. We repeated this procedure in a gradual manner until the door was completely closed and the reward out-of-reach. The two demonstrators had to open the box ten times in a row with the alternative technique blocked (within the second trial for Kalula: pull technique, and the third trial for Hull: slide technique; one session: five trials, trial duration: maximum 5 min, inter-trial interval: 15 min). Then, the birds had to open the box ten times in a row with the other option free (both demonstrators did so within their first trial session; one session: five trials, trial duration: maximum 5 min, inter-trial interval: 15 min).
Control group
To habituate the birds, we first placed one food reward in front of the box and waited until all birds approached the box and one subject ate the reward (‘pre-habituation’; trial duration: maximum 5 min, inter-trial interval: 2 h, eight trials in total). In order to show the birds that the box contained a food reward, we then placed one food reward inside the opened box, and repeated this procedure eight times in a row (‘habituation’; trial duration: maximum 5 min, inter-trial interval: 2 h). All naïve birds approached the box during these procedures: in pre-habituation, WitchyWitch got the reward on five occasions, and Raymond on three occasions. In the subsequent habituation, WitchyWitch got the reward on four occasions, Raymond on three occasions, and Adonsonia on one occasion.
Test phase
Test groups (pull group and slide group)
Social demonstrations took place in the opened compartment (pull group) or its location (slide group; see Subjects and Fig. S1, OSM). Before each test, the apparatus was placed against the aviary grid wall usually in the centre of the compartment (Fig. S1, OSM). To test ground-hornbills in an ecologically relevant social foraging context, we used an open diffusion experiment, where all subjects (the observers and the demonstrator) were simultaneously exposed to the rewarded apparatus during testing (Picard et al., 2017). Tests began when the experimenter opened the door (pull group) or left the test area (slide group). One minute minimum after each successful box opening, either by the demonstrator or the observer, birds were slightly forced to leave the compartment. The experimenter subsequently baited the box out of the view of subjects in the closed compartment (pull group), or underneath an opaque tissue covering the box (slide group).
Control group
The same procedure as that of the previous test groups was followed: the test began after the experimenter placed the apparatus and left the test area (the box could be opened using the slide/pull technique), except that the subjects had never seen a conspecific demonstrator opening the box before being tested.
For each experimental group (test groups and control group), ten tests were conducted in total (maximum two tests per day, each test between 20 and 25 min). Some observers (n = 4: pull group: Mud and Red rocks; slide group: Lindada and Makalali) saw both opening techniques (slide and pull) before their first opening success (for information about the number of social demonstrations received by observers before each of their opening success, see Fig. S2, OSM). This could not be prevented due to the social dynamic related to open diffusion experiments (all observers are simultaneously allowed to watch and learn from a trained demonstrator).
Scoring and analysis
All tests were live coded and video recorded and continuous second-by-second coding of the videos was performed. Using a coding scheme that included eight behavioural categories (Table S1, OSM), we counted for each bird the number of behaviours exhibited by assigning a score of 1 when the behaviour lasted at least 1 s (e.g., touching the apparatus) or was completed (e.g., opening the box by sliding/pulling). A second naïve observer (blind to the experimental condition) coded a random 20% of video recordings (six out of 30 tests in total in each of the three experimental groups). The Cohen’s kappa coefficient of inter-observer reliability calculated was κ = .92, which is considered high reliability.
Prediction 1: We expect learning enhancement to occur only within test groups
We implemented a generalised linear model (GLM; function: glm) using a Poisson error structure and a log link to assess whether there was a significant difference in learning enhancement (touching the box) between observer and control birds during the test phase. Based on a previous study examining social learning in a corvid species (Miller et al., 2016), our response variable (Touch) corresponded to the total number of times a ground-hornbill touched the box, and our explanatory variables included Trial number and Group (control/observer).
Prediction 2: We predict that only observers should be able to open the apparatus
We determined whether subjects that received (test groups) or did not receive social demonstrations (control group) succeeded in opening the box, and noted the trial number during which subjects opened the box for the first time in each group. We also used a Wilcoxon signed-rank test to assess whether the rapidity to first open the box differed between test groups. Opening success was defined as when subjects opened the box and got the reward, but also when birds did not get the reward, because (i) it has fallen accidentally at the bottom of the box, (ii) two subjects opened the box simultaneously using one technique, leaving one without a reward, or (iii) the reward was stolen (‘scrounged’; see Results).
Prediction 3: We expect subjects to show imitation learning
We assessed whether observers used the technique shown by the demonstrator (slide/pull) and touched more significantly specific parts of the box (pull parts 1–4 and slide part 5). We fitted a generalized linear mixed model (GLMM) with a negative binomial structure as overdispersion was reported (see below). Test (number of touches) was set as response variable, Location (pull parts 1, 2, 3, and 4, and slide part 5), Group (slide vs. pull), Sex (female vs. male), Social rank (subordinate, middle, dominant), as explanatory variables, and Subject (subject’s identity) as random effect. We did not include the variable Age (this species reaches maturity after 4 years; Kemp & Boesman, 2019), as all observers were adults in test groups. We also used Fisher’s exact test to assess individual preferences in using specific parts of the box at the individual level.
Prediction 4: We predict reporting agonistic and pilfering (‘scrounging’) behaviours within test groups
We counted the number of aggressive behaviours emitted and received by each subject (i.e., 21 aggressions and 22 received aggressions in total; see Results and Table 3) and the number of scrounging behaviours that occurred (observer or demonstrator) throughout the test phase (17 scrounging behaviours in total; see Results and Table 3). When scrounging occurs inside the box: one bird opens the box but another bird succeeds in inserting its beak rapidly within the box and in getting the food reward. By contrast, when scrounging occurs outside the box: one bird opens the box, removes the food reward out of the box, and then it gets stolen by another bird.
All analyses were conducted in R Version 3.6 (R Core Team, 2019) and we used the packages MuMIn for model selection (Bartón, 2009), lme4 for fitting models (Bates et al., 2015), and emmeans for post hoc tests (Lenth, 2020).
Results
Prediction 1: We expect learning enhancement to occur only within test groups
We carried out a GLM analysis to assess whether there were differences in learning enhancement between groups. The best-fitting model was explained by Group and confirmed our prediction: observers touched the box more significantly than naïve individuals (4.820 ± 1.002, z = 4.811, p < .001). During the test phase, all observers interacted with the apparatus while only one individual did so in the control group.
Prediction 2: We predict that only observers should be able to open the apparatus
We found evidence of social learning, as shown by the absence of variability in our data: all observers from the test groups succeeded in opening the box (see Video S1, OSM), while no naïve subject did so in the control group (albeit all birds from the control group approached and ate the food reward during habituation, and that one naïve individual (Adonsonia) touched the box during the test phase). Moreover, in 36 (pull group) and 39 (slide group) cases in total, observers and demonstrators from both groups re-opened the door just after another individual got the reward within the box (useful openings: 110 = pull group, and 132 = slide group). Subjects did not look or insert their beak inside the apparatus after this behaviour.
We used a Wilcoxon signed-rank test to assess potential differences between test groups regarding the rapidity (defined as test number) to first open the box. Although the pull technique was presented to the observers about twice as much as the slide technique (Kalula – pull group – showed 88 box openings, while Hull – slide group – exhibited 51 box openings; see Table 2), this did not affect the rapidity with which individuals first opened the box (Wilcoxon signed-rank test: p > 0.99; pull group: Mud = test 4, Nkosi = test 1, Red rocks = test 3; slide group: Chiredzi = test 1, Lindada = test 3, Makalali = test 3; Table 2).
Prediction 3: We expect subjects to show imitation learning
We carried out a GLMM analysis to assess imitation learning within test groups and Fisher’s exact test to detect potential preferences for specific parts of the box at the individual level.
Slide/pull technique
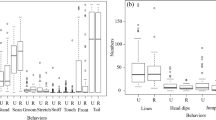
Only one bird in each test group (Red rocks = pull group and Lindada = slide group) used the method demonstrated initially (demonstrator Kalula: pull technique and demonstrator Hull: slide technique, respectively). Two other birds (Makalali = slide group and Mud = pull group) had a preference towards the alternate technique (the technique not shown by the trained demonstrator; Fig. 3).
Actions directed towards specific parts of the box (pull parts 1–4 and slide part 5)
The Poisson GLMM showed overdispersion (score: 2.24). We thus conducted a GLMM with a negative binomial error structure. The best-fitting model was explained by Social rank and Group (Akaike weight: 0.297; sum of weights: Social rank = 0.55 and Group = 0.47) and subsequent post hoc tests did not reveal significant differences (Table S2, OSM). At the individual level, however, we found that some individuals used significantly more than one part of the box (Fisher’s exact test, p = < .001). Indeed, the part of the door contacted over trials was consistent for three observers (pull group: Red rock = part 1, Mud = part 5; slide group: Lindada = part 5; Table 2).
Prediction 4: We predict reporting agonistic and pilfering (‘scrounging’) behaviours within test groups
We determined social rank of each individual and measured scrounging behaviours. Dominant observers (n = 2) monopolized the apparatus during the first two trials and were the first to open the box (slide group: Chiredzi = test 1; pull group: Nkosi = test 1; Table 3). Thirteen (pull group: two inside the box, 11 outside the box) and ten (slide group: two inside the box, eight outside the box) scrounging behaviours in total were observed throughout the experiment (Table 3). Scrounging did not interfere with the acquisition of food-finding behaviour as all observers succeeded in opening the box.
Discussion
This study is the first to investigate social learning in Bucerotiformes, an avian order that is still highly under-represented in the cognitive literature. Overall, our results show that observer ground-hornbills of both test groups rapidly learned a new food-finding behaviour from the observation of skilled conspecifics. Individuals from the control group were unable to solve the task, despite the interest of one individual contacting the apparatus. Only two individuals copied the technique and the directed action of the trained demonstrators. Details of the techniques used and the sites of the apparatus contacted to open the box are discussed as potential indicators of social learning mechanisms.
The fact that all observers, but one control subject, made contact with the box supports our first prediction about the ubiquitous presence of learning enhancement in birds (see Prediction 1 in Results; Nicol, 1995; Slagsvold & Wiebe, 2011). Similar results have been found in other avian species tested with different methodological procedures (e.g., Amazon parrots, Amazona amazonica: Picard et al., 2017; Eurasian jays, Garrulus glandarius: Miller et al., 2016; great-white pelicans, Pelecanus onocrotalus: Danel et al., 2020; jackdaws, Corvus monedula: Federspiel et al., 2019; New Caledonian crows, Corvus moneduloides: Logan et al., 2016; ravens, Corvus corax: Fritz & Kotrschal, 1999; white-throated magpie-jays, Calocitta Formosa: Langen, 1996; yellow-crowned bishops, Euplectes afer afer: Danel et al., 2018). Manipulations of the apparatus by the naïve subject from the control group might be attributed to its juvenile developmental stage. In birds confronted with social learning tasks, young individuals are usually more explorative and curious than adults (Biondi et al., 2013; Federspiel et al., 2019; Greenberg & Mettke-Hofmann, 2001).
Notably, within both test groups, all observers succeeded in opening the box (see Prediction 2 in Results). Each observer repeated box-opening behaviours throughout the tests, which indicates that the behaviour has been learned. Additional evidence for such reinforcement is that all birds, including demonstrators, had the tendency to re-open the ‘empty’ box (another individual already got the reward). No subject looked or inserted its beak inside the box after re-opening it, which allows us to preclude some misunderstanding of the task. Moreover, even though observers in each test group did not benefit from the same amount of demonstrations by trained individuals (88 in the pull group vs. 51 in the slide group), this did not seem to affect the rapidity with which subjects within the slide group solved the task (Table 2). By contrast, no subject from the control group succeeded in opening the box, although they all approached and got the reward within the opened box during habituation. The difference in social-learning performance between control and test birds may be attributed to the visual positive reinforcement of the food reward and the enhancing effect of the demonstrator during the test phase. Further social-learning tests, including measures on neophobia and motivational levels, should be conducted to draw firm conclusions.
Furthermore, ground-hornbills did not use the trained demonstrator technique significantly more than chance. However, at the individual level, and although they had encountered the opportunity to watch both methods prior to their first opening, four subjects exhibited preferences for one technique (Lindada = slide, Makalali = pull, Mud = slide, and Red rocks = pull; Fig. S2, OSM). These preferences emerged straight away without extensive manipulations of the box prior to the first success (Table 2). Interestingly, two birds (Red rocks and Lindada) opened the box by touching the same sites as their respective demonstrator. The possibility that these birds match (or imitate) the motor action of trained individuals, however, needs further investigation. Noteworthy, Red rocks used the alternate technique once. When this change in Red Rocks’s behaviour occurred, the access to part 1 of the door (which consisted of the usual part contacted by this subject to open the box) was blocked by a higher-ranked individual (Kalula). In this highly competitive context, and without prior experience with the alternate technique, Red rocks used the slide technique straight away and got the reward. We suggest that this subject acquired the slide technique through previous repeated observations, but flexibly adopted this optional strategy in constraining circumstances.
The fact that some observers watched both methods prior their first success, however, hinders our interpretations about the demonstrator’s influence on observers’ behaviours. To prevent the possibility for observers to watch both techniques, further experiments using the open diffusion approach may block the alternate technique specifically for demonstrators interacting with the box. This could be made, for instance, by fitting demonstrators with a leg ring containing a uniquely identifiable passive integrated transponder (PIT) tag combined with the use of automated boxes. An additional ghost control procedure (e.g., Kis et al., 2015), where two groups of ground-hornbills may observe different opening mechanisms automatically (e.g., a sliding door moving on either the left or the right side), could also be administered to know more about the social-learning mechanisms at play in this species (e.g., whether success is influenced by emulation of the movement of the object; Fugazza et al., 2019).
In contrast, two subjects (Chiredzi: slide group, Nkosi: pull group) used both techniques throughout the experiment and benefitted only from the demonstration of the trained technique prior to their first success. During the first two test trials, both guarded the apparatus and the other observers (and trained demonstrators) were prevented from entering and/or interacting with the experimental device. Therefore, the rapid performance of these subjects was most probably due to the fierce monopolization of the apparatus, rather than a difference in learning efficiency per se. Moreover, their first performance was not straightforward: Chiredzi and Nkosi manipulated the box by touching it or kicking it hard before opening it, which suggests local enhancement followed by individual trial-and-error learning.
Albeit scrounging did not inhibit subjects’ social learning performance, the role of the demonstrator’s age and social rank (including demonstrators and subsequent successful observers) on the technique of subordinate observers may not be excluded. The older and higher-ranked observers, Chiredzi and Nkosi, predominantly used the alternate technique (the technique not shown by the demonstrator) to solve the task. It is a possibility that the behaviour of the initial lower-ranked and younger demonstrators (Hull and Kalula, respectively) was not sufficiently salient to attract their attention. Age and high social status have been found to play a significant role in information transmission through social learning in animals (e.g., Eastern water skink, Eulamprus quoyii, Kar et al., 2017; domestic fowl, Gallus gallus domesticus, Nicol & Pope, 1999). Moreover, the identity and characteristics of the dominant demonstrator may also be important for the subordinates, which may display preferential attention to certain individual dominants (van de Waal et al., 2010). This may explain why the first successful technique of three observers (Lindada, Makalali, Mud) did not match the most frequently demonstrated method or was not influenced by the last demonstration they were exposed to. However, without a future experiment including subordinate demonstrators, this assumption must be taken with caution.
Contrary to other avian species tested on a similar open-diffusion paradigm (blue tits; Aplin et al., 2013, 2015), learning was not most frequently biased towards the demonstrator’s method. Our results resemble those obtained in another highly social and large-brained bird species, the Amazon parrot (Picard et al., 2017), where only individuals from the test groups learned efficiently how to open the box (although most parrots used both techniques to solve the task). It is a possibility that faithful copying is not ecologically relevant under natural condition for both species, and for several avian species in general (Nicol, 1995; Slagsvold & Wiebe, 2011). For instance, during snake pursuing and hunting in ground-hornbills, learning to focus the attention towards specific sites of the prey body (snake head) would be sufficient to kill it in all safety. In this context, the ground-hornbill observer may just need to locate where – rather than precisely how – the knowledgeable conspecific directs its pickaxe-like strikes on the prey. Thus, contrary to our second prediction, fine-scale stimulus enhancement may be sufficient to learn how to forage efficiently in young and inexperienced wild ground-hornbills.
Future experiments also need to be carried out to assess the influence of dominance and intrinsic individual features (age, body condition, motivation, personality, sex) on social learning in ground-hornbills. Similar to other communal avian breeders (e.g., babblers, Turdoides spp.; Florida scrub-jay, Aphelocoma coerulescens: Greenwood & Harvey, 1982; Paradis et al., 1998), sex differences in dispersal have been reported in ground-hornbills (Kemp, 1988). Generally, females disperse from their natal group at less than 1 year on average (Carstens et al., 2019), while males stay longer as helpers. Thus, regarding the challenges associated with dispersal (Aplin et al., 2013), testing females of varying developmental stages may provide information on whether they are more capable of attending to the behaviour of the demonstrator.
Importantly, these results can be exploited to develop relevant conservation tools (Greggor et al., 2014, 2017). A wide range of threats poses a significant risk to the persistence of Southern ground-hornbills in South Africa (Kemp et al., 2020; Morrison et al., 2005). Like other large birds, such as the Californian condor (Gymnogyps californianus; Kelly et al., 2015), several ground-hornbills end up being electrocuted when perching on transformer boxes (Taylor & Kemp, 2015). Management methods (e.g., altering structures) have had only a minimal impact on population protection. To promote successful reintroductions in the wild, a follow-up study is in progress to reduce this risky behaviour through social experience with conspecific demonstrators perching on mock transformer boxes and receiving a small but deterrent electrical shock that leads to an aversion to perching on transformer boxes post-reintroduction.
In conclusion, we report the first evidence of social learning in ground-hornbills within the foraging context. In the wild, the benefits of this cognitive capacity to this species are likely to involve several circumstances, varying from joining conspecifics at a food source to learning to kill dangerous prey such as highly venomous snakes. Given the consistent preferences of some observers for one specific technique and/or site of the box, it may be tempting to attribute our results to imitation learning. However, notably due to our small sample size, a more parsimonious explanation is that fine-grained learning enhancement most likely explains ground-hornbills’ performance.
References
Akins, C. K., & Zentall, T. R. (1996). Imitative learning in male Japanese quail (Coturnix japonica) using the two-action method. Journal of Comparative Psychology, 110, 316-320.
Akins, C. K., & Zentall, T. R. (1998). Imitation in Japanese quail: The role of reinforcement of demonstrator responding. Psychonomic Bulletin & Review, 5, 694-697.
Aplin, L. M., Farine, D. R., Morand-Ferron, J. & Sheldon, B. C. (2012). Social networks predict patch discovery in a wild population of songbirds. Proceedings of the Royal Society B: Biological Sciences, 279, 4199-4205.
Aplin, L. M., Sheldon, B. C., & Morand-Ferron, J. (2013). Milk bottles revisited: Social learning and individual variation in the blue tit, Cyanistes caeruleus. Animal Behaviour, 85, 1225-1232.
Aplin, L. M., Farine, D. R., Morand-Ferron, J., Cockburn, A., Thornton, A., Sheldon, B. C. (2015). Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature, 518, 538-541.
Bartón, K. (2009). MuMIn: Multi-model inference, R package version 0.12. 0. http://r-forge.r-project.org/projects/mumin/.
Bates, D., Mächler, M., Bolker, B., Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67, 1-48.
Benson-Amram, S., Heinen, V. K., Gessner, A., Weldele, M. L., & Holekamp, K. E. (2014). Limited social learning of a novel technical problem by spotted hyenas. Behavioural Processes, 109, 111-120.
Biondi, L. M., García, G. O., Bó, M. S., & Vassallo, A. I. (2010). Social learning in the Caracara Chimango, Milvago chimango (Aves: Falconiformes): An age comparison. Ethology, 116, 722-735.
Biondi, L. M., Guido, J., Madrid, E., Bó, M. S., & Vassallo, A. I. (2013). The effect of age and sex on object exploration and manipulative behavior in a Neotropical raptor, the Chimango Caracara, Milvago chimango. Ethology, 119, 221-232.
Boinski, S. (1999). The social organizations of squirrel monkeys: Implications for ecological models of social evolution. Evolutionary Anthropology, 8, 101-112.
Bouchard, J., Goodyer, W., & Lefebvre, L. (2007). Social learning and innovation are positively correlated in pigeons (Columba livia). Animal Cognition, 10, 259-266.
Brown, G. R., & Fawcett, T. W. (2005). Sexual selection: Copycat mating in birds. Current Biology, 15, R626-R628.
Cadieu, N., Fruchard, S. & Cadieu, J. C. 2010. Innovative individuals are not always the best demonstrators: Feeding innovation and social transmission in Serinus canaria. PLoS One, 5, e8841.
Campbell, F. M., Heyes, C. M., & Goldsmith, A. R. (1999). Stimulus learning and response learning by observation in the European starling in a two-object/two-action test. Animal Behaviour, 58, 151-158.
Canteloup, C., Hoppitt, W., & van de Waal, E. (2020). Wild primates copy higher-ranked individuals in a social transmission experiment. Nature communications, 11, 1-10.
Carstens, K. F., Kassanjee, R., Little, R. M., Ryan, P. G., & Hockey, P. A. (2019). Natal dispersal in the Southern Ground Hornbill Bucorvus leadbeateri. Ostrich, 90, 119-127.
Claidiere, N., Messer, E. J., Hoppitt, W., & Whiten, A. (2013). Diffusion dynamics of socially learned foraging techniques in squirrel monkeys. Current Biology, 23, 1251-1255.
Coussi-Korbel S, Fragaszy, D. M. (1995). On the relation between social dynamics and social learning Animal Behaviour, 50, 1441-1453.
Custance, D., Prato-Previde, E., Spiezio, C., Rigamonti, M. M., & Poli, M. (2006). Social learning in pig-tailed macaques (Macaca nemestrina) and adult humans (Homo sapiens) on a two-action artificial fruit. Journal of Comparative Psychology, 120, 303-313.
Danel, S., van Buuren, M., von Bayern, A. M., & Osiurak, F. (2018). Male yellow-crowned bishops (Euplectes afer afer) acquire a novel foraging behaviour by social learning. Journal of Ethology, 37, 235- 239.
Danel, S., von Bayern, A. M., & Osiurak, F. (2019). Ground-hornbills (Bucorvus) show means-end understanding in a horizontal two-string discrimination task. Journal of Ethology, 37, 117–122.
Danel, S., Troina, G., Dufour, V., Bailly-Bechet, M., von Bayern, A. M., & Osiurak, F. (2020). Social learning in great white pelicans (Pelecanus onocrotalus): A preliminary study. Learning & Behavior, 48, 344-350.
Dawson, B. V., & Foss, B. M. (1965). Observational learning in Budgerigars. Animal Behaviour, 13, 470–474.
Dindo, M., Thierry, B., & Whiten, A. (2008). Social diffusion of novel foraging methods in brown capuchin monkeys (Cebus apella). Proceedings of the Royal Society B: Biological Sciences, 275, 187–193.
Farrar, B. G., Ostojic, L., & Clayton, N. (2021). The hidden side of animal cognition research: Scientists’ attitudes toward bias, replicability and scientific practice. PloS one, 16, e0256607.
Fawcett, T. W., Skinner, A. M., & Goldsmith, A. R. (2002). A test of imitative learning in starlings using a two-action method with an enhanced ghost control. Animal Behaviour, 64, 547-556.
Federspiel, I. G., Boeckle, M., von Bayern, A. M. P., & Emery, N. J. (2019). Exploring individual and social learning in jackdaws (Corvus monedula). Learning & behavior, 47, 258-270.
Fritz, J., & Kotrschal, K. (1999). Social learning in common ravens, Corvus corax. Animal Behaviour, 57, 785-793.
Fritz, J., Bisenberger, A., & Kotrschal, K. (2000). Stimulus enhancement in greylag geese: Socially mediated learning of an operant task. Animal Behaviour, 59, 1119-1125.
Fugazza, C., Petro, E., Miklósi, Á., & Pogány, Á. (2019). Social learning of goal-directed actions in dogs (Canis familiaris): Imitation or emulation? Journal of Comparative Psychology, 133, 244-251.
Gajdon, G. K., Fijn, N., & Huber, L. (2004). Testing social learning in a wild mountain parrot, the kea (Nestor notabilis). Animal Learning & Behavior, 32, 62-71.
Galef, B. G. (1992). The question of animal culture. Human nature, 3, 157-178.
Galef, B. G., Jr., Manzig, L. A., & Field, R. M. (1986). Imitation learning in budgerigars: Dawson and Foss (1965) revisited. Behavioral Processes, 13, 191-202.
Gariépy, J. F., Watson, K. K., Du, E., Xie, D. L., Erb, J., Amasino, D., & Platt, M. L. (2014). Social learning in humans and other animals. Frontiers in Neuroscience, 8, 58.
Giraldeau, L. A., Lefebvre, L. (1987). Scrounging prevents cultural transmission of food-finding behaviour in pigeons. Animal Behaviour, 35, 387-394.
Goulson, D., Park, K. J., Tinsley, M. C., Bussière, L. F., & Vallejo-Marin, M. (2013). Social learning drives handedness in nectar-robbing bumblebees. Behavioral Ecology and Sociobiology, 67, 1141-1150.
Greco, B. J., Brown, T. K., Andrews, J. R., Swaisgood, R. R., & Caine, N. G. (2013). Social learning in captive African elephants (Loxodonta africana africana). Animal Cognition, 16, 459-469.
Greenberg, R. & Mettke-Hofmann, C. (2001). Ecological aspects of neophobia and neophilia in birds. Current Ornithology, 16, 119-169.
Greenwood, P. J., & Harvey, P. H. (1982). The natal and breeding dispersal of birds. Annual Review of Ecology and Systematics, 13, 1-21.
Greggor, A. L., Clayton, N. S., Phalan, B., & Thornton, A. (2014). Comparative cognition for conservationists. Trends in Ecology & Evolution, 29, 489-495.
Greggor, A. L., Thornton, A., & Clayton, N. S. (2017). Harnessing learning biases is essential for applying social learning in conservation. Behavioral Ecology and Sociobiology, 71, 16.
Greggor, A. L., Blumstein, D. T., Wong, B. B., & Berger-Tal, O. (2019a). Using animal behavior in conservation management: A series of systematic reviews and maps. Environmental Evidence, 8, 1–3.
Greggor, A. L., Price, C. J., & Shier, D. M. (2019b). Examining the efficacy of anti-predator training for increasing survival in conservation translocations: A systematic review protocol. Environmental Evidence, 8, 1-9.
Griffin, A. S. (2004). Social learning about predators: A review and prospectus. Animal Learning & Behavior, 32, 131-140.
Guttridge, T. L., van Dijk, S., Stamhuis, E. J., Krause, J., Gruber, S. H., & Brown, C. (2013). Social learning in juvenile lemon sharks, Negaprion brevirostris. Animal Cognition, 16, 55-64.
Kelly, T. R., Rideout, B. A., Grantham, J., Brandt, J., Burnett, L. J., Sorenson, K. J., ... & Johnson, M. (2015). Two decades of cumulative impacts to survivorship of endangered California condors in California. Biological Conservation, 191, 391-399.
Heyes, C. M., & Dawson, G. R. (1990). A demonstration of observational learning in rats using a bidirectional control. The Quarterly Journal of Experimental Psychology Section B, 42, 59–71.
Hunt, G. R., Holzhaider, J. C., & Gray, R. D. (2012). Prolonged parental feeding in tool-using New Caledonian crows. Ethology, 118, 423-430.
Inoue-Nakamura, N., Matsuzawa, T. (1997). Development of stone tool use by wild chimpanzees (Pan troglodytes). Journal of Comparative Psychology, 111, 159-173.
Kappeler, P. M. (1987). The acquisition process of a novel behavior pattern in a group of ring-tailed lemurs (Lemur catta). Primates, 28, 225-228.
Kar, F., Whiting, M. J., & Noble, D. W. (2017). Dominance and social information use in a lizard. Animal Cognition, 20, 805-812.
Kaiser, D. H., Zentall, T. R., & Galef Jr, B. G. (1997). Can imitation in pigeons be explained by local enhancement together with trial-and-error learning? Psychological Science, 8, 459–460.
Kemp, A. C. (1988). The behavioural ecology of the Southern Ground Hornbill: Are competitive offspring at a premium? In: van den Elzen R, Schuchmann K-L, Schmidt-Koenig K (eds), Current topics in avian biology: Proceedings of the International Centennial Meeting of the Deutsche Ornithologen-Gesellschaft. Deutsche Ornithologen-Gesellschaft. pp 261-271.
Kemp, L. (2017). Southern Ground-Hornbill (Bucorvus leadbeateri) Husbandry Manual. PAAZATM African Preservation Programme. Editor: J. Meyer, Montecasino Bird Gardens South Africa, 1–73.
Kemp, A. C. & Boesman, P. (2019). Southern Ground-hornbill (Bucorvus leadbeateri). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions. https://www.hbw.com/node/55881. Accessed 8 June 2019.
Kemp, A.C. & Kemp, M. (1980). The biology of the Southern Ground Hornbill Bucorvus leadbeateri (Vigors) (Aves: Bucerotidae). Annals of the Transvaal Museum 32, 65-100.
Kemp, L. V., Kotze, A., Jansen, R., Dalton, D. L., Grobler, P., & Little, R. (2020). Review of trial reintroductions of the long-lived, cooperative breeding Southern Ground-hornbill. Bird Conservation International, 30, 533–558.
Kendal, R. L., Boogert, N. J., Rendell, L., Laland, K. N., Webster, M., & Jones, P. L. (2018). Social learning strategies: Bridge-building between fields. Trends in Cognitive Sciences, 22, 651-665.
Kis, A., Huber, L., & Wilkinson, A. (2015). Social learning by imitation in a reptile (Pogona vitticeps). Animal Cognition, 18, 325-331.
Knight, G. M. (1990). A preliminary investigation into the status, distribution and some aspects of the foraging ecology of the Southern ground hornbill (Bucorvus cafer) in Natal. MSc dissertation, University Natal, Durban.
Laland, K. N., & Hoppitt, W. (2003). Do animals have culture? Evolutionary Anthropology: Issues, News, and Reviews: Issues, News, and Reviews, 12, 150-159.
Langen, T. A. (1996). Social learning of a novel foraging skill by white-throated magpie-jays (Calocitta formosa, Corvidae): A field experiment. Ethology, 102, 157-166.
Lefebvre, L. (2000). Feeding innovations and their cultural transmission in bird populations. The evolution of cognition, 311-328.
Lefebvre, L., & Bouchard, J. (2003). Social learning about food in birds. In The biology of traditions: Models and evidence (ed. D. M. Fragaszy & S. Perry), pp. 94–126. Cambridge, UK: Cambridge University Press.
Lefebvre, L., Whittle, P., Lascaris, E., & Finkelstein, A. (1997). Feeding innovations and forebrain size in birds. Animal Behaviour, 53, 549-560.
Lenth, R. (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.8. https://cran.r-project.org/package=emmeans
Logan, C. J., Breen, A. J., Taylor, A. H., Gray, R. D., & Hoppitt, W. J. (2016). How New Caledonian crows solve novel foraging problems and what it means for cumulative culture. Learning & Behavior, 44, 18-28.
McLean, I. G., Hölzer, C., & Studholme, B. J. (1999). Teaching predator-recognition to a naive bird: Implications for management. Biological Conservation, 87, 123-130.
Miller, R., Logan, C. J., Lister, K., & Clayton, N. S. (2016). Eurasian jays do not copy the choices of conspecifics, but they do show evidence of stimulus enhancement. PeerJ, 4, e2746.
Morrison, K., Daly, B., Burden, D., Engelbrecht, D., Jordan, M., Kemp, A., Kemp, M., Potgieter, C., et al. (2005). PHVA Southern Ground Hornbill (Bucorvus leadbeateri) Population and Habitat Viability Assessment workshop report. Johannesburg, South Africa.
Nicol, C. J. (1995). The social transmission of information and behaviour. Applied Animal Behaviour Science, 44, 79-98.
Nicol, C. J., & Pope, S. J. (1999). The effects of demonstrator social status and prior foraging success on social learning in laying hens. Animal Behaviour, 57, 163–171.
Paradis, E., Baillie, S. R., Sutherland, W. J. & Gregory, R. D. (1998). Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology, 67, 518-536.
Picard, A. M., Hogan, L., Lambert, M. L., Wilkinson, A., Seed, A. M., & Slocombe, K. E. (2017). Diffusion of novel foraging behaviour in Amazon parrots through social learning. Animal Cognition, 20, 285-298.
R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL https://www.R-project.org/.
Reader, S. M. & Biro, D. (2010). Experimental identification of social learning in wild animals. Learning & Behavior, 38, 265-283.
Ricklefs, R. E. (2004). The cognitive face of avian life histories: the 2003 Margaret Morse Nice lecture. The Wilson Journal of Ornithology, 116, 119-133.
Rørvang, M. V., Christensen, J. W., Ladewig, J., & McLean, A. (2018). Social learning in horses-fact or fiction? Frontiers in Veterinary Science, 5, 1-8.
Sargeant, B. L., & Mann, J. (2009). From social learning to culture: Intrapopulation variation in bottlenose dolphins. The question of animal culture, 152-173.
Schnöll, A. V. (2010). Testing for social diffusion of two novel foraging skills and conformity in wild redfronted lemurs (Eulemur fulvus rufus) (Doctoral dissertation, uniwien).
Slagsvold, T., & Wiebe, K. L. (2011). Social learning in birds and its role in shaping a foraging niche. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 969-977.
Spence, K. W. (1937). Experimental studies of learning and higher mental processes in infra- human primates. Psychological Bulletin, 34, 806- 850.
Taylor, M.R., Kemp, L.V. (2015). Southern Ground Hornbill. In: Taylor M, Peacock F, Wanless R (eds), The 2015 Eskom red data book of birds of South Africa, Lesotho and Swaziland. . pp. 119-121.
Tennie, C., Call, J., & Tomasello, M. (2006). Push or pull: Imitation vs. emulation in great apes and human children. Ethology, 112, 1159-1169.
Thornton, A., & Clutton-Brock, T. (2011). Social learning and the development of individual and group behaviour in mammal societies. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 978-987.
Thorpe, W. H. (1963). Learning and Instinct in Animals. 2nd edn. Methuen.
Urbanek, R. P., Fondow, L. E., Zimorski, S. E., Wellington, M. A., & Nipper, M. A. (2010). Winter release and management of reintroduced migratory Whooping Cranes Grus americana. Bird Conservation International, 20, 43–54.
van de Waal, E., Renevey, N., Favre, C. M., and Bshary, R. (2010). Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proceedings or the Royal Society of London Series B: Biological Sciences, 277, 2105-2111.
van de Waal, E., & Whiten, A. (2012). Spontaneous emergence, imitation and spread of alternative foraging techniques among groups of vervet monkeys. PLoS One, 7, e47008.
van Leeuwen, E. J., Cronin, K. A., & Haun, D. B. (2014). A group-specific arbitrary tradition in chimpanzees (Pan troglodytes). Animal Cognition, 17, 1421-1425.
Whiten, A., & Mesoudi, A. (2008). Establishing an experimental science of culture: Animal social diffusion experiments. Philosophical Transactions of the Royal Society B: Biological Sciences, 363, 3477-3488.
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., Tutin, C. E. G., & Boesch, C. (1999). Cultures in chimpanzees. Nature, 399, 682-685.
Wyness, W. (2011). Home range use by Southern Ground-Hornbills (Bucorvus leadbeateri)-quantifying seasonal habitat selection and vegetation characteristics (Doctoral dissertation, University of Cape Town).
Zajonc, R. B. (1965). Social facilitation. Science, 149, 269-274.
Zentall, T. R. (2004). Action imitation in birds. Animal Learning & Behavior, 32, 15-23.
Zentall, T. R., Sutton, J., & Sherburne, L. M. (1996). True imitative learning in pigeons. Psychological Science, 7, 343-346.
Zoghby, B. A., Ryan, P. G., Little, R. M., Reid, T., & Hockey, P. A. (2015). Seasonal changes in movement and habitat use by Southern Ground-Hornbills in the South African Lowveld. Ostrich, 86, 87-95.
Acknowledgements
This work was supported by grants from the European Union and Région Provence Alpes Côte d’Azur (PACA, FRANCE) within the program FAJE 2019/2020 (to SD). We thank the Mabula Ground-Hornbill Project (Mabula, South Africa), Mpumalanga Tourism and Parks Agency Loskop Dam Nature Reserve, and National Zoological Gardens (Pretoria, South Africa) for allowing us to do this study. We are very grateful to Florian van Huffel for help in building the apparatus and training compartments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiment was approved by the SANBI NZG Research Ethics and Scientific Committee (RESC, permit number: SANBI NZG/RES/P19/14).
Conflicts of interests
The authors declare that they have no conflicts of interest.
Additional information
Open practices statement
The data or materials for the experiments reported here are available from the corresponding author on reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1406 kb)
Rights and permissions
About this article
Cite this article
Danel, S., Rebout, N. & Kemp, L. Social diffusion of new foraging techniques in the Southern ground-hornbill (Bucorvus leadbeateri). Learn Behav 51, 153–165 (2023). https://doi.org/10.3758/s13420-022-00518-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-022-00518-4