Abstract
Neural plasticity in the hippocampus has been studied in a wide variety of model systems, including in avian species where the hippocampus underlies specialized spatial behaviours. Examples of such behaviours include navigating to a home roost over long distances by homing pigeons or returning to a potential nest site for egg deposit by brood parasites. The best studied example, however, is food storing in parids and the interaction between this behaviour and changes in hippocampus volume and neurogenesis. However, understanding the interaction between brain and behaviour necessitates research that includes studies with at least some form of captivity, which may itself affect hippocampal plasticity. Captivity might particularly affect spatial specialists where free-ranging movement on a large scale is especially important in daily, and seasonal, behaviours. This review examines how captivity might affect hippocampal plasticity in avian spatial specialists and specifically food-storing parids, and also considers how the effects of captivity may be mitigated by researchers studying hippocampal plasticity when the goal is understanding the relationship between behaviour and hippocampal change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The link between plasticity of brain and behaviour, and more specifically how changes in each affect the other, is an ongoing and seemingly limitless area of research. Neural plasticity can occur at many levels: systems reorganization, such as compensatory hypertrophy between neural pathways with different levels of input, volume change of specific brain regions within a pathway, neurogenesis and recruitment of new neurons to functional regions, and molecular changes at the level of neurotransmitter quantity and number of receptors on the cell membrane (Brenowitz & Larson, 2015; Sweatt, 2016; Tramontin & Brenowitz, 2000). While studying neural plasticity during development is critical for understanding the ontogeny of the brain and how it sets up an organism for functioning as an adult, behavioural and neural plasticity also occurs in adults—usually in response to changes in some aspect of an animal’s environment—in order to better function in a particular context (Lambert et al., 2019).
Adult neural plasticity has been studied in a wide variety of model systems, including Aplysia, rodents, and primates, usually with the goal of understanding what factors may enhance or inhibit plasticity. However, the use of laboratory studies necessitates the use of at least some form of captivity—whether life-long or only while manipulating variables of interest. While captivity allows for increased experimental control of conditions (i.e., to isolate specific effects of interest from confounding variables), captivity itself may also affect behaviour and the brain. The degree to which captivity affects the model may vary; in species such as mice that are bred specifically for laboratory work, transition to captivity is not a concern, but a mouse bred for the laboratory may have a diminished, yet untestable, potential for plasticity compared with free-living counterparts that experience many more demands, pressures, and the need to constantly adapt to their environment. In species that are free-living, it is especially important to understand how the transition to, and time held in, captivity affects both the brain and behaviour, to separate the effects of a manipulation from the potentially confounding effects of captivity.
Hippocampal neural plasticity in food-storing birds may be an especially valuable paradigm in providing insight into the effects of captivity on the brain-behaviour interaction. This is because unlike Aplysia, rodents, or even primates that are studied almost exclusively in captivity, food-storing bird species may be studied in both in the wild (i.e., in their natural habitat) and in the laboratory. Because there is extensive literature on neural plasticity in food-storing birds, using both field-based and laboratory-based approaches, and even some research that directly compares wild and captive populations, studies of food-storing birds, and indeed other spatial specialists (e.g., long-distance migrants and brood parasites), provide insight on how captivity may specifically affect the study of the brain and behaviour in general. This paper, in honour of Dr. David Sherry, will review findings on hippocampal plasticity in avian spatial specialists, including some previously unpublished data examining whether conditions of captivity, housing type and housing location, affect a specific measure of neural plasticity, hippocampal neurogenesis.
Food-storing and other spatial paradigms: Background
Probably the best studied spatial specialists are species that cache food for later use, a feat that relies not on chance, but on memory of hundreds of individual locations, visited for a very short time when the food is stored, and remembered days to weeks later (Sherry & Hoshooley, 2007) when food is retrieved for consumption. These species include parids (e.g., black-capped chickadees, Poecile atricapillus) as well as corvids (e.g., Clark’s nutcrackers, Nucifraga columbiana; Balda & Kamil, 1992; Bednekoff et al., 1997; Krebs et al., 1989; Odum, 1942; Sherry et al., 1992). However, our understanding of spatial behaviour in birds extends beyond food caching species and includes navigation in homing pigeons (Columba livia), who are able to return to a home roost from novel locations and in long-distance migrants (e.g., semipalmated sandpipers, Calidris pusilla) that travel semiannually between breeding and wintering grounds (Sherry & MacDougall-Shackleton, 2015). Another example is brood parasitism in cowbirds (genus Molothrus), where females search for and remember the location of potential nest sites of other species in which to lay their eggs (Guigueno & Sherry, 2017).
Associated with each of these increased demands on spatial memory is the specialization of neural structures that support spatial behaviour, including the hippocampus (Sherry et al., 1992). Evidence of neural specialization can appear in many forms: hippocampi of migratory white-crowned sparrows (Zonotrichia leucophrys gambelii) are, relative to their telencephalon, larger than those of non-migratory sparrows (Z. l. nuttallii; Pravosudov et al., 2006); adult homing pigeons with hippocampal lesions have difficulty finding a direct path to their home roost (Bingman et al., 1984), and inactivation of hippocampal NMDA receptors (the activity of which is critical for establishing long-term potentiation) during acquisition of a spatial learning task interferes with the ability to remember the location of food (Shiflett et al., 2004). These differences between specialists and non-specialists (e.g., Lange et al., 2021) appear to be relatively stable, as captivity does not seem to eliminate them (e.g., Hoshooley & Sherry, 2007; see also Pravosudov & Smulders, 2010). However, hippocampal plasticity seems to be dramatically affected by captivity. Factors associated with captivity such as spatial confinement, reduced activity level, and social isolation—possibly all contributing to increase stress—may all affect the hippocampus, and in particular, hippocampal neurogenesis. In this section, we examine more carefully how captivity has been considered in studies of hippocampal plasticity in food-storing and other spatial specialists.
Hippocampus volume plasticity and captivity
A gross measure of neural plasticity is that of volume, specifically whether the volume of a particular brain region of interest varies across conditions. A related measure is neuron number within the region; a combination of neuron number and region volume allows estimate of changes in neural density and may provide more information on the nature of volume change (e.g., Tramontin et al., 1998)
One of the first studies to examine hippocampal neural plasticity in food-storing birds looked at seasonal variation in rates of food-storing and hippocampus volume in black-capped chickadees. Smulders et al. (1995) reported that chickadees captured in October had a larger hippocampus by volume compared with those captured in winter (December, February), spring (April) or summer (June, August), coinciding with increased rates of storing seed in October compared with other times of year (Sherry & Hoshooley, 2007; but see Pravosudov, 2006). Similar results have recently been found in wild-caught willow tits (Poecile montanus): hippocampus volume peaked in September when their storing behaviour also peaked (Lange et al., 2021).
Studies in black-capped chickadees subsequent to Smulders et al. (1995) have not always been able to replicate this seasonal variation (Sherry & MacDougall-Shackleton, 2015). However, there is a consistent difference between these subsequent studies and the results of Smulders et al. (1995): length of time in captivity. Both Smulders et al. (1995) and Lange et al. (2021) sacrificed birds the same day of capture. In contrast, Hoshooley and Sherry (2004) housed birds indoors in cages for 1–2 weeks with free access to food before sacrifice, and Hoshooley et al. (2007) housed birds outdoors in cages for 1 week with free access to food and to multiple short sessions where they were given the opportunity to store and retrieve seeds. Captivity was a necessary part of the design in these two studies—the researchers were interested not only in seasonal volume changes but also in hippocampal neurogenesis; using the cell birth marker 5-bromo-2'-deoxyuridine (BrdU) require time for the dividing cells to migrate to the hippocampus (Balthazart & Ball, 2014; see next section). However, in doing this, it may have compromised their ability to detect any change in hippocampus volume.
Another study with black-capped chickadees held in captivity (Hoshooley & Sherry, 2007) did detect a difference in hippocampus volume between birds captured in fall (October and November) and in spring (February to April) rather than across multiple seasons. There may be several reasons why it was possible to detect the seasonal effect in this particular study: First, collapsing birds into two groups (instead of across multiple time points) lent more statistical power than in other studies. Second, they housed birds in outdoor aviaries rather than in cages and/or indoors, potentially mitigating effects of spatial restriction in cages. Finally, waiting 6 weeks (rather than 1–2 weeks as in Hoshooley & Sherry, 2004, and Hoshooley et al., 2007) for sacrifice may have given birds time to habituate to the stress of captivity.
As stated before, laboratory studies are useful in controlling extraneous variables and manipulating specific variables of interest; in food-storing birds, this could be photoperiodic condition or opportunity to store food, to determine which may have a stronger effect on neural plasticity. In a laboratory study that manipulated breeding condition by exposing black-capped chickadees to different photoperiods (MacDougall-Shackleton et al., 2003), there was no variation in hippocampus volume, even though birds exposed to short days (as in fall) stored more seeds (and had smaller gonads) than birds exposed to long days (as in spring), indicating that although photoperiod was efficacious in changing both behaviour and physiology, it did not stimulate volume change in the hippocampus. Other researchers have facilitated or restricted food-storing to determine the effect of the behaviour itself on hippocampus volume: willow tits (Cristol, 1996) and mountain chickadees (Poecile gambeli; Pravosudov et al., 2002) that were able to store food while in captivity did not have a larger hippocampus volume or increased hippocampal neuron density. Taken together, these studies indicate that moving wild birds into captivity may specifically suppress volume change in the hippocampus even when they are given the opportunity to store food. Further to this, hand-reared marsh tits (Poecile palustris) that had experience storing over development had larger hippocampi and more hippocampal neurons as adults than those that did not (Clayton & Krebs, 1994), meaning that hippocampus volume plasticity is possible in captive birds, as long as they are accustomed to captivity from early on, rather than transition to captivity from the wild as adults.
Volume has also been used as a measure of hippocampal plasticity in other spatial specialists, although less extensively than in food-storing species. In brood parasitic cowbirds, females, not males, remember locations of various potential host nests to monitor progress of nest-building and egg-laying to determine the best time to deposit their own egg (Guigueno & Sherry, 2017). Shiny cowbirds (Molothrus bonariensis) and screaming cowbirds (M. rufoaxillaris), both brood parasites, sacrificed immediately after capture have a larger relative hippocampus when in breeding condition compared with nonbreeding condition (Clayton et al., 1997). Although this appears to be evidence of seasonal plasticity of hippocampus volume, the breeding and nonbreeding groups from Clayton et al. (1997) were processed in two different batches, and the difference could, therefore, be due to a batch effect (Guigueno et al., 2016) rather than a true seasonal difference. In brown-headed cowbirds (M. ater), hippocampus volume did not change with breeding condition in either males or females (Guigueno et al., 2016); these birds were captured, transported approximately 2 hours from the capture site to the laboratory, and held overnight before sacrifice. It seems unlikely that transport and minimal time in captivity eliminated any possible hippocampus volume difference between breeding and nonbreeding cowbirds, and instead that hippocampus volume is seasonally stable in this particular species.
Day et al. (2008) examined how time in captivity, and specifically the lack of opportunity to search for and remember host nests, affected hippocampus volume in brown-headed cowbirds. Males and females were captured in the breeding season and held in an outdoor aviary for up to 10 days or 1 year, and a third group was held for a year in an outdoor aviary and then in an isolation chamber for 5 weeks prior to sacrifice. In female cowbirds, hippocampus volume was reduced in the two groups of birds housed for a year or more compared with the group held for 10 days or less. There was no reduction in male hippocampus as males do not find and remember host nests, and thus do not possess a specialized hippocampus (Guigueno & Sherry, 2017). This research seems to indicate that in female cowbirds, long-term captivity reduces hippocampus volume compared with short-term captivity. Further research is needed to determine whether it is reduced compared with birds not held at all in captivity, whether this varies seasonally, and whether access to host nests mitigates this reduction.
There is even less research on hippocampus volume changes in navigational specialists, such as migratory species or homing pigeons (Mehlhorn & Rehkämper, 2009; Sherry & MacDougall-Shackleton, 2015). In one study of a migratory songbird, garden warblers (Sylvia borin) captured just prior to migration and held in captivity for 5 days or 1 year showed no difference in hippocampus volume—either indicating that any amount of captivity affects hippocampus volume, or that the hippocampus of these birds is resistant to its negative effects (Healy et al., 1996). In semipalmated sandpipers, a long-distance migratory shorebird, the hippocampus does show seasonal variation: volume was larger in wintering (i.e., already migrated) than migrating birds (i.e., actively migrating; de Morais Magalhães et al., 2017); these birds were not held in captivity at all. Homing pigeons given developmental experience navigating to a home loft had larger hippocampi than those that did not (Cnotka et al., 2008). Finally, dark-eyed juncos (Junco hyemalis), a non-food-storing species with a large territory, held in captivity had smaller hippocampus volume than free-living counterparts, regardless of home range size (Smulders et al., 2000). None of these studies, however, even together, can tell us whether captivity eliminates seasonal change in hippocampus volume of navigational specialists.
Studying hippocampal neurogenesis in captivity
While volume has historically been the measure of choice to quantify gross neuroanatomical change in food-storing birds specifically, other measures may be more sensitive in assessing hippocampal plasticity relative to spatial behaviour (Roth et al., 2010) and may be particularly susceptible to effects of captivity. One of these is neurogenesis—the proliferation, differentiation, migration, recruitment, and integration of new neurons in the central nervous system (Zhao et al., 2008). Neural stem cells proliferate in specific regions of the brain, and through a host of genetic, molecular, and environmental factors (Doetsch & Scharff, 2001; Gonçalves et al., 2016) these cells develop into mature neurons sporting structural and functional characteristics necessary for successful integration to neural circuits. In songbirds, the primary site of proliferation is the subventricular zone (SVZ) of the lateral ventricle. Cells then migrate away from the SVZ and are recruited to regions as needed. Finally, mature neurons are integrated into existing neural circuits (Barnea & Pravosudov, 2011; Pytte, 2016). However, not all neurons are incorporated, and thus it is important to consider not only the rate of cell birth in the SVZ but also the rate of recruitment, survival, and incorporation into functional regions such as the nuclei of the vocal control system (Gahr et al., 2002), auditory perceptual regions (Pytte et al., 2010), and the hippocampus (LaDage, 2016).
There is ample evidence that hippocampal neurogenesis is important for, and related to, the specialization of spatial memory (see Table 1 for a comprehensive list). There is increased hippocampal neurogenesis in spatial compared with non-spatial specialists: migratory species have more hippocampal neurogenesis than non-migratory species (Barkan et al., 2014; LaDage et al., 2011) and food-storing species have more than non-food-storing species (Hoshooley & Sherry, 2007). Further, in brood parasites (e.g., brown-headed cowbirds; shiny cowbirds) females, who remember locations of various potential host nests, have more hippocampal neurogenesis than males (Guigueno & Sherry, 2017).
Particularly well-studied is the relationship between food-storing behaviour and neurogenesis, with respect to how the frequency of food storing and retrieval changes seasonally. In their seminal work, Barnea and Nottebohm (1994) found that black-capped chickadees captured in October experienced an increase in neuron recruitment to the hippocampus (i.e., increased neurogenesis in this region) compared with birds captured in various months across other seasons. This peak in hippocampal neurogenesis coincided with a presumed increase in food-storing behaviour, suggesting that neuron recruitment to the hippocampus was tied to forming new spatial memories. Barnea and Nottebohm were perhaps ahead of their time in their study design by incorporating direct comparison of captive and free-living black-capped chickadees that had been administered [3H]-thymidine ([3H]-TdR) as a cell birth marker. Birds in the captive group were taken from the wild in the spring and fall and held in outdoor aviaries (where they were able to store seeds) for 2 months prior to [3H]-TdR administration. Birds in the free-living group (n = 74) were captured at various times of year, given the cell birth maker in one dose, and released back into the wild. Some were recaptured 6 weeks later; others were recaptured well after 6 weeks. The remaining birds that were administered [3H]-TdR and released (43%) were never recaptured, perhaps illustrating why this study design is used infrequently. Nevertheless, when matched for time of year, captive birds had a 50% reduction in new cell survival in the hippocampus compared with recaptured free-living birds, and there was no difference in hippocampus volume. Corroborating this result, mountain chickadees held in captivity had less hippocampal neurogenesis than free-living birds (LaDage et al., 2010). These results seem to support the idea that captivity suppresses neuronal survival in the hippocampus of food-storing birds.
Results from subsequent studies, also using chickadees captured at various times of year, have not replicated the October peak in neuron recruitment found by Barnea and Nottebohm (1994)—but as with hippocampus volume, captivity may explain this difference. As described above, Hoshooley and Sherry (2004) captured birds at the peak of storing (October and November) and retrieval (January and February/March), and Hoshooley et al. (2007) captured chickadees at regular intervals across the annual cycle: October, January, April, and July. Neither study found an October peak in hippocampal recruitment in October, and in fact, the peak for Hoshooley et al. (2007) was in January (possibly indicating that increased neurogenesis was related to retrieving, rather than storing, seeds). In both studies, birds were held in captivity for 1–2 weeks before sacrifice, and if captivity suppresses neurogenesis, as observed in Barnea and Nottebohm (1994), it may have obscured any October peak in neuron recruitment to the hippocampus. Differences in cell birth markers (e.g., [3H]-thymidine vs. BrdU) or study timelines (6 weeks vs. 1–2 weeks between marker administration to sacrifice) may also have contributed to differences in results between studies.
Amounts of food-storing and neuron recruitment appear to be related: black-capped chickadees that live in harsher environments, and presumably need to store food more frequently, have greater neuron recruitment to the hippocampus than those that experience less harsh environments and thus have less need to store (Chancellor et al., 2011; Roth et al., 2011). This difference persists even when these birds are brought into the laboratory and hand-reared (i.e., before experiencing variation in harsh environmental conditions): black-capped chickadees that were captured from a harsh climate stored more and had higher hippocampal neuron recruitment than chickadees captured from a less harsh climate and held in the same conditions (Roth et al., 2012). Opportunity to store and retrieve in captivity can also affect neuron recruitment to the hippocampus. Although wild-caught mountain chickadees had reduced recruitment compared with birds in the same study not held in captivity at all, birds in the captive groups that were allowed to store and retrieve seeds had more cells recruited to the hippocampus compared with birds not allowed to store and retrieve (LaDage et al., 2010). If food-storing behaviour and recruitment of new neurons to the hippocampus are strongly related, and the opportunity to store can partially mitigate the suppressing effects of captivity, it is somewhat curious that Hoshooley et al. (2007) did not observe this peak: Their wild-caught chickadees were given an opportunity to store and retrieve food, and the most storing was observed in October. Perhaps the amount of storing that did occur in these captive birds was not sufficient to mitigate the effects of captivity.
Neurogenesis and food-storing behaviour are linked, but neurogenesis may be affected specifically by transition to captivity. Patel et al. (1997) also observed greater neuron recruitment to the hippocampus in hand-reared marsh tits given the opportunity to store compared with those who were restricted from performing this behaviour. However, these birds were hand-reared in the laboratory from 12 days posthatch (Clayton, 1996), meaning they experienced being captured well before experimental measures, and they did not have experience with food-storing in the wild, and thus neurogenesis was not affected by those events.
Can size and location of housing mitigate effects of captivity?
Housing birds in captivity not only reduces the need and pressure, if food is available freely, to perform ecologically relevant behaviours such as food storing and retrieval, but changes the physical space in which birds live. Studies vary greatly on length of time in captivity preceding the study, overall duration of the captive study, and housing size (i.e., individual cages or aviaries) and location (i.e., indoor or outdoor; see Table 1). It is possible that housing birds in large aviaries outdoors, where birds have more space and experience natural photoperiodic and weather conditions, partially mitigates the effects of captivity. For example, one reason that Smulders et al. (1995) and Hoshooley and Sherry (2007) may have been able to detect a peak in hippocampus volume was because they housed captive birds in outdoor aviaries, whereas other studies housed birds indoors in cages (Hoshooley & Sherry, 2004) or outdoors in cages (Hoshooley et al., 2007).
In a study designed specifically to examine the effects of captivity on hippocampal plasticity in food-storing birds, Tarr et al. (2009) captured black-capped chickadees, injected them with the cell birth marker BrdU, and divided them into two groups: one that was released back into the wild for 4-6 weeks and recaptured, and another that was housed in indoor cages for the same amount of time. Hippocampus volume was reduced by 23% after 4–6 weeks of captivity compared with free-living counterparts, but contrary to the findings of Barnea and Nottebohm (1994), hippocampal neurogenesis (i.e., number of BrdU+ cells) was not reduced in the captive black-capped chickadees.
The difference between Barnea and Nottebohm (1994) and Tarr et al. (2009) may be explained by the time of year the birds were captured for each study. Barnea and Nottebohm (1994) detected suppression of neurogenesis in captive birds caught during their peak in neurogenesis in October. However, Tarr et al. (2009) captured birds in late November and early December, when the purported fall peak in neurogenesis might have tapered off (but see Hoshooley et al., 2007). Thus, the Tarr et al. (2009) results could be due to a floor effect of neurogenesis level, meaning that the base rate of neurogenesis in these birds may have been low enough that the effect of captivity could not reduce the number of cells any further. If Barnea and Nottebohm (1994) removed birds captured in October from their analyses, the effect of captivity on neurogenesis might be, like Tarr et al. (2009), below the threshold for detection. This is corroborated by data from LaDage et al. (2009), who found a difference in hippocampal neurogenesis between captive mountain chickadees that stored compared with captive birds that did not: These birds were captured in September, closer to the October time point used by Barnea and Nottebohm (1994), than to the November and December time point used by Tarr et al. (2009).
These three studies also differ in housing conditions of captive birds. In both the Tarr et al. (2009) and the LaDage et al. (2009) studies, captive birds were housed in indoor cages, while those in the Barnea and Nottebohm (1994) study were housed in outdoor aviaries. The former studies therefore imposed not only a decrease in space but also removal from the natural environment. Together, these results suggest that different aspects of captivity may differentially affect hippocampal neurogenesis. We have collected data from a small number of birds that seems to support the idea that size, but not location of housing, affects neurogenesis. Below we outline the methods we used and results of this exploratory dataset.
We captured adult black-capped chickadees on Dalhousie University campus and private property (with landowner permission) in Halifax, Nova Scotia, Canada. All birds were captured using baited Potter traps or mist nets at three times of the year in 2013 and 2015: fall (November–December; prewinter solstice); winter (February, prespring equinox), spring (May–June; presummer solstice). We transported birds to our facilities at Dalhousie University within 1 hour of capture and assigned individuals to one of four housing conditions (using block randomization): outdoor aviary, outdoor cage, indoor aviary, or indoor cage. Outdoor aviaries were individual 3.3 × 2.1 × 2.5 m enclosures made of metal alloy with wire mesh framing and were located on the roof of the Psychology Department in the Life Sciences Centre at Dalhousie University. Aviaries were equipped with a cylindrical nest tube made of PVC tubing partially filled with wood chips, with large tree branches (natural), and with an artificial Christmas tree (2 m tall, 654 branch points). They also contained a plastic free-standing birdbath and an assortment of small plastic food and water cups affixed to the Christmas tree branches and to the sides of the aviary. Outdoor cages were individual 0.62 × 0.32 × 0.32 m cages positioned within an otherwise empty aviary on the roof. Each cage was fitted with a small cylindrical nest tube partially filled with wood chips, three wooden perches, a small birdbath, and food and water cups affixed to the sides of the cage. Birds in both indoor groups were housed in the same aviaries or cages with identical setups to the outdoor housing groups. Indoor bird rooms were located on the first floor of the Psychology wing of the Life Sciences Centre. Rooms were maintained on a natural light–dark cycle (updated weekly), based on the light–dark cycle in Halifax, Nova Scotia, Canada. Room temperatures ranged from 19–21°C regardless of time of the year. All birds in all groups received food and water ad libitum.
After a 24-hour acclimation period, birds were given four 0.05 cc intramuscular injections of 1.5 mg/mL BrdU in 0.1M phosphate buffered saline (PBS, pH = 7.5) at 2-hour intervals, as described by Hoshooley and Sherry (2004); however, we report only data with doublecortin (DCX) staining here. Exactly 6 weeks after BrdU injections, birds were sacrificed via overdose, transcardially perfused, sex confirmed via gonads (testes or ovaries), and brain tissue collected and cryoprotected before freezing (as per standard protocol; e.g., Roach et al., 2016) until sectioning at 30 μm and processing. We labeled hippocampal neurogenesis using DCX immunoreactivity (DCX-ir; 1° Ab sc-8066, Santa Cruz Biotechnology Inc., RRID:AB_2088494) following immunohistochemical methods similar to those used by Hall and MacDougall-Shackleton (2012) and Wada et al. (2014). Mounted and coverslipped tissue was imaged with an Olympus U-TVO 5XC-3 camera mounted on an Olympus BX51 microscope at 40× magnification (400× total magnification). Similar to Hall and MacDougall-Shackleton (2012) and Wada et al. (2014), we quantified neurogenesis by measuring the percentage of microscope field of view covered by DCX-stained cell bodies, neurites, and axons (%DCX+ coverage) using the “Threshold” feature in ImageJ. We measured %DCX+ coverage in the ventral hippocampus (Hp1), dorsomedial hippocampus (Hp2), and a control region (HA, hyperpallium apicale), located using a stereotaxic atlas of the songbird brain (Nixdorf-Bergweiler & Bischof, 2007).
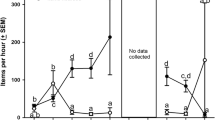
A total of 17 chickadee brains were processed for our analyses: six captured in fall, three captured in winter, and eight captured in spring. In this set of birds there was no effect of season in any region—Hp1: F(2, 11) < 2; Hp2: F(2, 11) = 3.17, p = .08; HA: F(2, 10) < 2—so we show data based on housing condition (see Fig. 1): outdoor aviary (n = 5), outdoor cage (n = 2), indoor aviary (n = 4), indoor cage (n = 6). Birds housed in cages had lower %DCX+ coverage in one region of the hippocampus (Hp2; dorsomedial hippocampus) compared with those housed in aviaries, F(1, 13) = 9.08, p < .01, ω2 = .32 (see bottom-left panel), but there was no difference between birds housed indoors or outdoors—Hp1 and Hp2: Fs(1, 13) < 2; HA: F(1, 12) < 1. Although we cannot tell by how much neurogenesis was reduced compared with free living birds as in Tarr et al. (2009), LaDage et al. (2009), and Barnea and Nottebohm (1994), our data suggest that a reduction in space (i.e., individual cage versus aviary) affects neurogenesis more than location of housing (i.e., indoor versus indoors).
Housing type affects hippocampal neurogenesis in black-capped chickadees. Birds (N = 17) were captured from the wild and housed in one of four conditions: indoor cage, outdoor cage, indoor aviary, outdoor aviary. After 6 weeks, we measured neuronal survival in two regions of the hippocampus (ventral hippocampus, Hp1; dorsomedial hippocampus, Hp2) and one control region (hyperpallium apicale, HA) using doublecortin (DCX) immunohistochemistry (top-right panel). We quantified the percentage of the microscope field of view covered by DCX-stained cell bodies, neurites, and axons (%DCX+ coverage). Asterisk (*) indicates a significant (p < .01) effect of housing type on %DCX+ coverage in the dorsomedial hippocampus. Dots indicate data from individual birds.
Mitigation of effects of captivity should perhaps focus on size of housing (i.e., aviaries rather than cages), over location. A possible explanation why larger cages are beneficial could be differences in amount of activity between birds housed in cages, who can only fly very short amounts between closely placed perches, and birds housed in aviaries, who can, by comparison, move more freely. Exercise upregulates neurogenesis in mammalian hippocampus of animals in impoverished environments (van Praag, 2008), and one study in European starlings (Sturnus vulgaris), a non-food-storing, nonmigrant species, found some evidence of increased neurogenesis in hippocampus in birds given the opportunity to fly in a wind tunnel, but only under poor diet conditions (Hall et al., 2014). More research is needed to determine if activity levels, or even access to exercise, is why birds housed in aviaries do not have the same level of suppression of neurogenesis as birds housed in cages.
Mechanisms underlying captivity-induced changes in the brain
How does captivity affect hippocampal plasticity in studies of food-storing birds? Different measures, such as neurogenesis and volume, may be affected differently by the transition to, and time in captivity. Changes can occur much more quickly at the cellular level than at the systems level, thus it seems plausible that neurogenesis as a measure of plasticity may be more acutely affected by captivity than volume. There is some evidence for this: neurogenesis, but not volume, was reduced in captive birds compared with those released and recaptured (Tarr et al., 2009). Further, volume was not negatively correlated with time in captivity when birds were held less than 10 days (Day et al., 2008). Since cellular change typically precedes system change, we focus on neurogenesis here, as it seems particularly susceptible to suppression from transition to captivity, especially compared with volume.
It is possible that captivity suppresses neurogenesis overall. As stated above, birds housed in the laboratory experience an impoverished environment compared with free-living counterparts; indoors there is a lack of daily temperature fluctuations or changes in humidity, cloud cover, or barometric pressure (LaDage et al., 2010; Patel et al., 1997). If housed individually, or even with a small number of conspecifics in the same room, birds may not interact with familiar mates or flock members, nor hear the same number of conspecifics (or heterospecifics) typically heard by free-living birds—however, number and sex of housing companions does not affect neurogenesis in captive birds (Fox et al., 2010). Furthermore, birds in captivity are typically given free access to food and water, meaning foraging (let alone the need for food-storing) is not required. Impoverished environments have long been associated with suppression in neurogenesis in rodents (van Praag et al., 2000) and birds (Smulders, 2017). This means that studies of captive birds may be working with a smaller range of neurogenesis, and any differences that may exist between groups would be much harder to detect—thus, researchers should minimize time in captivity (none at all if possible), or housing birds in the most natural conditions if possible. In fact, some studies seem to acknowledge this by eliminating any captivity in study design, particularly those examining seasonal or environmental change (Chancellor et al., 2011; Guigueno et al., 2016; Roth & Pravosudov, 2009).
Underlying, or in addition to, a relatively impoverished captive environment is the stress associated with transitioning into and holding of free-living birds in captivity. The hippocampus is an integral part of the hypothalamic-pituitary-adrenal (HPA) axis and stress response: after release of the stress hormone corticosterone in response to an acute stressor, glucocorticoid receptors in the hippocampus aid with the shut-down of the stress response (Smulders, 2021). Prolonged stress can lead to chronic elevation of circulating corticosterone, and in turn can suppress neurogenesis in the avian hippocampus (Smulders, 2017) and other brain regions (Honarmand et al., 2016). However, mountain chickadees implanted with corticosterone did not have reduced hippocampal neurogenesis compared with controls (Pravosudov & Omanska, 2005). That said, these birds had been held in captivity for 4 months; being habituated to the captive environment might have overshadowed any differences from the manipulation of corticosterone.
It is possible there is an ideal window during which to conduct neurogenesis studies on captive birds: after a period of habituation to captivity to minimize effects of acute stress due to transition to the lab, and before any longer-term effects of an impoverished environment or chronic stress. Wild caught house sparrows (Passer domesticus), removed from the wild and placed into captivity, show an increase in baseline levels of corticosterone (Love et al., 2017). In a follow-up study, DuRant et al. (2020) quantified the expression of neurohormones involved in the stress response (corticotropin releasing hormone, CRH; gonadotropic inhibitory hormone, GnIH) as well as the expression of the glucocorticoid receptor (GR). They found increased expression of CRH and GR mRNA in the hypothalamus of sparrows kept in captivity for 24 and 45 days. At 66 days of captivity, the quantity of GR mRNA did not differ from wild-captured birds, suggesting that birds were able to acclimate to captivity between 45 and 66 days of captivity. However, waiting this long for habituation may not be possible if a particular stage of neurogenesis (proliferation, migration, survival) is under study. More research is required to understand the interaction between the transition to captivity, length of time in captivity, and stage of hippocampal neurogenesis, but it seems likely that stress and acclimation to captivity to mitigate stress should be considered when designing timelines of experiments.
In order to fully understand the mechanisms of how captivity affects neural plasticity in the hippocampus of spatial specialists, it is necessary to move beyond the systems level (i.e., volumetric changes) and consider both cellular (e.g., morphological change) and subcellular (e.g., gene expression) changes. Further, we should consider data from non-spatial specialists and non-avian models. For example, Roth et al. (2017) examined how different types of captivity affected neuronal morphology in the hippocampus of house sparrows, a non-spatial specialist. House sparrows were captured from the wild and either sacrificed immediately or transferred into one of two captive treatments: aviary or individual cage. In addition to showing that birds in both captive treatments had a smaller hippocampus by volume compared with the wild group, they also found that both captive groups had significantly smaller total dendritic lengths compared to wild birds. They also found effects of captivity on overall dendritic spine density in the hippocampus: birds who spent the entirety of the experiment in the aviary condition had reduced dendritic spine density compared with wild birds, and birds who spent the entirety of the experiment (4 months) in small individual cages had a decreased spine density compared with the aviary birds. To probe at the directionality of the relationship, a subset of birds was removed from the aviary after 2 months and transferred into the individual cages; birds were also removed from individual cages and transferred into aviaries. Interestingly, birds in the aviary group who were transferred to individual cages did not show a reduction in hippocampal spine density; but those housed in small cages and transferred into the aviary did show an increase in spine density. These results align with the exploratory data we present in Fig. 1: the more captive space is reduced, the greater effect on hippocampal plasticity.
More recently, George et al. (2020) found that zebra finches (Taeniopygia guttata), a non-spatial specialist, had reduced expression of brain-derived neurotrophic factor (BDNF) in auditory perceptual regions (caudomedial nidopallium, caudomedial mesopallium, Field L) after being removed from an aviary and placed into isolation chambers for relatively short periods of captivity (overnight to 2 days). Further, they determined that the suppression of BDNF expression was due to alteration of the methylation profile of the BDNF gene. This work may point to a critical mechanism for the interaction between hippocampal plasticity and stress after reduction in size of housing condition, a change particularly relevant to spatial specialists. Expression of BDNF is critical for cell growth and neurogenesis (Brenowitz & Larson, 2015; Zhao et al., 2008) and is upregulated in concurrence with plasticity (Thompson et al., 2012), so any suppression of BDNF expression would consequently reduce neurogenesis. Alterations to epigenetic markers (e.g., changes in DNA methylation, histone modifications) in response to stress occur over a relatively short period of time (Gray et al., 2017; Stankiewicz et al., 2013), such as the time it takes to capture a bird from the wild and transport it to the laboratory. Thus, the mechanism by which stress from the transition to captivity can almost immediately suppress hippocampal plasticity could be through an epigenetic mechanism, such as altered methylation of genes like BDNF. There is extensive research on how stress alters BDNF expression through epigenetic mechanisms in the hippocampus of rodents (Fuchikami et al., 2010); however, because sequencing for the BDNF gene is not yet complete in most bird species, and is particularly lacking in spatial specialists, we cannot yet test this idea directly. However, it is likely epigenetic markers play a significant role in the stress interacts with mechanisms of neural plasticity, resulting in suppression of neurogenesis among other measures.
Acknowledging the limits of captivity
In making the assumption that the laboratory is an impoverished and stressful environment in which to study neurogenesis, researchers sometimes attempt to reduce stress and mitigate effects of captivity where possible. To reduce stress induced by specific methods, some researchers choose to use doublecortin (DCX), a microtubule-associated protein that is expressed in new neurons up to a month postmitosis, to quantify neurogenesis (Balthazart et al., 2008). Using this endogenous marker means use of injections used to administer an exogenous marker, such as BrdU, is eliminated. DCX is a valid and valuable alternative to BrdU and does not require the introduction of additional stressors, such as handling and injections, multiple times in a study (Balthazart & Ball, 2014). As recommended above, enriching the environment through maximizing space and possibly some natural cues from being outdoors may also mitigate the stress of transition to captivity (LaDage et al., 2010; Patel et al., 1997). However, other enrichment measures may not be effective. For example, mountain chickadees housed in various social groups (e.g., individually, same sex pairs, mixed sex pairs, mixed sex set of four) did not differ in hippocampal neurogenesis (Fox et al., 2010).
The value of captive studies cannot be underestimated: laboratory-based and field-based studies have complementary strengths and weaknesses (Calisi & Bentley, 2009). From the most basic perspective of research design, manipulating variables of interest while controlling others is essential for understanding how particular aspects of the environment may affect neurogenesis and overall plasticity. It also means researchers can minimize variability in experience among subjects over the course of a study to more easily detect differences between groups experiencing different conditions. However, at the most extreme level of experiential control, where animals are reared in captivity, there seems to be a difference between the brain and behaviour of captive-reared and wild-reared animals (Kruska, 1988). For example, wild Norway rats have increased cell proliferation and neuron survival in the dentate gyrus (i.e., a site of constant cell proliferation in the mammalian brain) relative to one strain of laboratory-bred rats (Sprague-Dawley, but not Long Evans or Norway Brown), there were no differences in the cell proliferation or the number of new neurons in the hippocampus of wild and captive rats; although some differences were apparent in juveniles (Epp et al., 2010). Although rearing animals in captivity may eliminate the stress due to transition from the wild, the potential for neural plasticity in these lab-reared animals may be reduced.
This is where studying hippocampal neurogenesis in wild-caught spatial specialists, and in particular, food-storing birds, offers an advantage. In captivity, spatial specialists may be offered the opportunity to perform hippocampal-related behaviours that enrich their experience in the laboratory and complement natural abilities. Cowbirds can perform spatial memory tasks on a touch screen (e.g., Guigueno et al., 2014; Sherry & Guigueno, 2019) and pigeons can be given a landmark spatial task in the aviary (e.g., Balda & Wiltschko, 1995). In these two types of specialists, researchers can leverage the potential of a specialized hippocampus to perform spatial behaviours in the laboratory. However, these are not naturalistic spatial behaviours: cowbirds cannot not search for and remember locations of nests to parasitize, and pigeons cannot be given a navigational task, because both of these natural spatial behaviours occur on a scale not possible to replicate in captivity. In contrast, food-storing birds can perform the exact spatial task they would perform in the wild—storing and retrieving food from that location after a delay. The food-storing paradigm therefore offers a way to study hippocampal neurogenesis in captive wild birds while incorporating ecologically relevant behaviours, which in turn seems to mitigate effects of captivity. In combination with studies of free-living birds, the study of captive food-storing birds offers a unique model to investigate the effects of captivity on neurogenesis, in addition to which factors promote and inhibit other measures of hippocampal plasticity. Further, it is important to acknowledge the limitations of captive studies in models beyond avian spatial specialists—in both captured and captive-raised study species. In this way we can more fully understand the mechanisms of plasticity in the hippocampus using both laboratory-based and field-based approaches.
Data Availability
Data and materials generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Balda, R. P., & Kamil, A. C. (1992). Long-term spatial memory in Clark’s nutcracker, Nucifraga columbiana. Animal Behaviour, 44(4), 761–769. https://doi.org/10.1016/S0003-3472(05)80302-1
Balda, R. P., & Wiltschko, W. (1995). Spatial memory of homing pigeons, Columba livia, tested in an outdoor aviary. Ethology, 100(3), 253–258. https://doi.org/10.1111/j.1439-0310.1995.tb00329.x
Balthazart, J., & Ball, G. F. (2014). Endogenous versus exogenous markers of adult neurogenesis in canaries and other birds: Advantages and disadvantages. Journal of Comparative Neurology, 522(18), 4100–4120. https://doi.org/10.1002/cne.23661
Balthazart, J., Boseret, G., Konkle, A. T. M., Hurley, L. L., & Ball, G. F. (2008). Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. European Journal of Neuroscience, 27(4), 801–817. https://doi.org/10.1111/j.1460-9568.2008.06059.x
Barkan, S., Yom-Tov, Y., & Barnea, A. (2014). A possible relation between new neuronal recruitment and migratory behavior in Acrocephalus warblers. Developmental Neurobiology, 74(12), 1194–1209. Scopus. https://doi.org/10.1002/dneu.22198
Barnea, A., & Nottebohm, F. (1994). Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proceedings of the National Academy of Sciences of the United States of America, 91(23), 11217–11221. https://doi.org/10.1073/pnas.91.23.11217
Barnea, A., & Pravosudov, V. (2011). Birds as a model to study adult neurogenesis: Bridging evolutionary, comparative and neuroethological approaches. European Journal of Neuroscience, 34(6), 884–907. https://doi.org/10.1111/j.1460-9568.2011.07851.x
Bednekoff, P. A., Balda, R. P., Kamil, A. C., & Hile, A. G. (1997). Long-term spatial memory in four seed-caching corvid species. Animal Behaviour, 53(2), 335–341. https://doi.org/10.1006/anbe.1996.0395
Bingman, V. P., Bagnoli, P., Ioalè, P., & Casini, G. (1984). Homing behavior of pigeons after telencephalic ablations. Brain, Behavior and Evolution, 24(2/3), 94–108. https://doi.org/10.1159/000121308
Brenowitz, E. A., & Larson, T. A. (2015). Neurogenesis in the Adult Avian Song-Control System. Cold Spring Harbor Perspectives in Biology, 7(6). https://doi.org/10.1101/cshperspect.a019000
Calisi, R. M., & Bentley, G. E. (2009). Lab and field experiments: Are they the same animal? Hormones and Behavior, 56(1), 1–10. https://doi.org/10.1016/j.yhbeh.2009.02.010
Chancellor, L. V., Roth, T. C., LaDage, L. D., & Pravosudov, V. V. (2011). The effect of environmental harshness on neurogenesis: A large-scale comparison. Developmental Neurobiology, 71(3), 246–252. https://doi.org/10.1002/dneu.20847
Clayton, N. S. (1996). Development of food-storing and the hippocampus in juvenile marsh tits (Parus palustris). Behavioural Brain Research, 74(1/2), 153–159. https://doi.org/10.1016/0166-4328(95)00049-6
Clayton, N. S., & Krebs, J. R. (1994). Hippocampal growth and attrition in birds affected by experience. Proceedings of the National Academy of Sciences, 91(16), 7410–7414. https://doi.org/10.1073/pnas.91.16.7410
Clayton, N. S., Reboreda, J. C., & Kacelnik, A. (1997). Seasonal changes of hippocampus volume in parasitic cowbirds. Behavioural Processes, 41(3), 237–243. https://doi.org/10.1016/S0376-6357(97)00050-8
Cnotka, J., Möhle, M., & Rehkämper, G. (2008). Navigational experience affects hippocampus size in homing pigeons. Brain, Behavior and Evolution, 72(3), 233–238. https://doi.org/10.1159/000165102
Cristol, D. A. (1996). Food storing does not affect hippocampal volume in experienced adult willow tits. Behavioural Brain Research, 81(1/2), 233–236. Scopus. https://doi.org/10.1016/S0166-4328(96)89083-8
Day, L. B., Guerra, M., Schlinger, B. A., & Rothstein, S. I. (2008). Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater obscurus). Behavioral Neuroscience, 122(3), 527–534. https://doi.org/10.1037/0735-7044.122.3.527
de Morais Magalhães, N. G., Diniz, C. G., Diniz, D. G., Henrique, E. P., Moraes, I. A. M., De Melo, M. A. D., Sherry, D. F., & Picanço Diniz, C. W. (2017). Hippocampal neurogenesis and volume in migrating and wintering semipalmated sandpipers (Calidris pusilla). PLOS ONE, 12(6). https://doi.org/10.1371/journal.pone.0179134
Doetsch, F., & Scharff, C. (2001). Challenges for brain repair: Insights from adult neurogenesis in birds and mammals. Brain, Behavior and Evolution, 58(5), 306–322. https://doi.org/10.1159/000057572
DuRant, S., Love, A. C., Belin, B., Tamayo-Sanchez, D., Santos Pacheco, M., Dickens, M. J., & Calisi, R. M. (2020). Captivity alters neuroendocrine regulators of stress and reproduction in the hypothalamus in response to acute stress. General and Comparative Endocrinology, 295, 113519. https://doi.org/10.1016/j.ygcen.2020.113519
Epp, J. R., Haack, A. K., & Galea, L. A. M. (2010). Task difficulty in the Morris water task influences the survival of new neurons in the dentate gyrus. Hippocampus, 20(7), 866–876. https://doi.org/10.1002/hipo.20692
Fox, R. A., Roth, T. C., II, LaDage, L. D., & Pravosudov, V. (2010). No effect of social group composition or size on hippocampal formation morphology and neurogenesis in mountain chickadees (Poecile gambeli). Developmental Neurobiology, 70(7), 538–547. https://doi.org/10.1002/dneu.20795
Fuchikami, M., Yamamoto, S., Morinobu, S., Takei, S., & Yamawaki, S. (2010). Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investigation, 7(4), 251–256. https://doi.org/10.4306/pi.2010.7.4.251
Gahr, M., Leitner, S., Fusani, L., & Rybak, F. (2002). What is the adaptive role of neurogenesis in adult birds? In: Progress in Brain Research (Vol. 138, pp. 233–254). Elsevier. https://doi.org/10.1016/S0079-6123(02)38081-6
George, J. M., Bell, Z. W., Condliffe, D., Dohrer, K., Abaurrea, T., Spencer, K., Leitão, A., Gahr, M., Hurd, P. J., & Clayton, D. F. (2020). Acute social isolation alters neurogenomic state in songbird forebrain. Proceedings of the National Academy of Sciences, 117(38), 23311–23316. https://doi.org/10.1073/pnas.1820841116
Gonçalves, J. T., Schafer, S. T., & Gage, F. H. (2016). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell, 167(4), 897–914. https://doi.org/10.1016/j.cell.2016.10.021
Gray, J. D., Kogan, J. F., Marrocco, J., & McEwen, B. S. (2017). Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nature Reviews Endocrinology, 13(11), 661–673. https://doi.org/10.1038/nrendo.2017.97
Guigueno, M. F., & Sherry, D. F. (2017). Hippocampus and spatial memory in brood parasitic cowbirds. In M. Soler (Ed.), Avian brood parasitism: Behaviour, ecology, evolution and coevolution (pp. 203–218). Springer. https://doi.org/10.1007/978-3-319-73138-4_11
Guigueno, M. F., Snow, D. A., MacDougall-Shackleton, S. A., & Sherry, D. F. (2014). Female cowbirds have more accurate spatial memory than males. Biology Letters, 10(2), 20140026. https://doi.org/10.1098/rsbl.2014.0026
Guigueno, M. F., MacDougall-Shackleton, S. A., & Sherry, D. F. (2016). Sex and seasonal differences in hippocampal volume and neurogenesis in brood-parasitic brown-headed cowbirds (Molothrus ater). Developmental Neurobiology, 76(11), 1275–1290. https://doi.org/10.1002/dneu.22421
Hall, Z. J., & MacDougall-Shackleton, S. A. (2012). Influence of testosterone metabolites on song-control system neuroplasticity during photostimulation in adult European starlings (Sturnus vulgaris). PLOS ONE, 7(7), e40060. https://doi.org/10.1371/journal.pone.0040060
Hall, Z. J., Bauchinger, U., Gerson, A. R., Price, E. R., Langlois, L. A., Boyles, M., Pierce, B., McWilliams, S. R., Sherry, D. F., & MacDougall-Shackleton, S. A. (2014). Site-specific regulation of adult neurogenesis by dietary fatty acid content, vitamin E and flight exercise in European starlings. European Journal of Neuroscience, 39(6), 875–882. https://doi.org/10.1111/ejn.12456
Healy, S. D., Gwinner, E., & Krebs, J. R. (1996). Hippocampal volume in migratory and non-migratory warblers: Effects of age and experience. Behavioural Brain Research, 81(1), 61–68. https://doi.org/10.1016/S0166-4328(96)00044-7
Honarmand, M., Thompson, C. K., Schatton, A., Kipper, S., & Scharff, C. (2016). Early developmental stress negatively affects neuronal recruitment to avian song system nucleus HVC. Developmental Neurobiology, 76(1), 107–118. https://doi.org/10.1002/dneu.22302
Hoshooley, J. S., & Sherry, D. F. (2004). Neuron Production, Neuron Number, and Structure Size Are Seasonally Stable in the Hippocampus of the Food-Storing Black-Capped Chickadee (Poecile atricapillus). Behavioral Neuroscience, 118(2), 345–355. https://doi.org/10.1037/0735-7044.118.2.345
Hoshooley, J. S., & Sherry, D. F. (2007). Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Developmental Neurobiology, 67(4), 406–414. https://doi.org/10.1002/dneu.20316
Hoshooley, J. S., Phillmore, L. S., Sherry, D. F., & MacDougall-Shackleton, S. A. (2007). Annual cycle of the black-capped chickadee: Seasonality of food-storing and the hippocampus. Brain, Behavior and Evolution, 69(3), 161–168. https://doi.org/10.1159/000096984
Krebs, J. R., Sherry, D. F., Healy, S. D., Perry, V. H., & Vaccarino, A. L. (1989). Hippocampal specialization of food-storing birds. Proceedings of the National Academy of Sciences of the United States of America, 86(4), 1388–1392. https://doi.org/10.1073/pnas.86.4.1388
Kruska, D. (1988). Mammalian domestication and its effect on brain structure and behavior. In H. J. Jerison & I. Jerison (Eds.), Intelligence and evolutionary biology (pp. 211–250). Springer. https://doi.org/10.1007/978-3-642-70877-0_13
LaDage, L. D. (2016). Factors that modulate neurogenesis: A top-down approach. Brain, Behavior and Evolution, 87(3), 184–190. https://doi.org/10.1159/000446906
LaDage, L. D., Roth, T. C., II, Fox, R. A., & Pravosudov, V. V. (2009). Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behavioral Neuroscience, 123(2), 284–291. https://doi.org/10.1037/a0014817
LaDage, L. D., Roth, T. C., II, Fox, R. A., & Pravosudov, V. V. (2010). Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proceedings of the Royal Society B: Biological Sciences, 277(1684), 1071–1079. https://doi.org/10.1098/rspb.2009.1769
LaDage, L. D., Roth, T. C., II, & Pravosudov, V. V. (2011). Hippocampal neurogenesis is associated with migratory behaviour in adult but not juvenile sparrows (Zonotrichia leucophrys ssp.). Proceedings of the Royal Society B: Biological Sciences, 278(1702), 138–143. https://doi.org/10.1098/rspb.2010.0861
Lambert, K., Eisch, A. J., Galea, L. A. M., Kempermann, G., & Merzenich, M. (2019). Optimizing brain performance: Identifying mechanisms of adaptive neurobiological plasticity. Neuroscience and Biobehavioral Reviews, 105, 60–71. https://doi.org/10.1016/j.neubiorev.2019.06.033
Lange, H., Walker, L., Orell, M., & Smulders, T. V. (2021). Seasonal changes in the hippocampal formation of hoarding and non-hoarding tits. Learning and Behavior. https://doi.org/10.3758/s13420-021-00481-6
Love, A. C., Lovern, M. B., & DuRant, S. E. (2017). Captivity influences immune responses, stress endocrinology, and organ size in house sparrows (Passer domesticus). General and Comparative Endocrinology, 252, 18–26. https://doi.org/10.1016/j.ygcen.2017.07.014
MacDougall-Shackleton, S. A., Sherry, D. F., Clark, A. P., Pinkus, R., & Hernandez, A. M. (2003). Photoperiodic regulation of food storing and hippocampus volume in black-capped chickadees, Poecile atricapillus. Animal Behaviour, 65(4), 805–812. https://doi.org/10.1006/anbe.2003.2113
Mehlhorn, J., & Rehkämper, G. (2009). Neurobiology of the homing pigeon—A review. Naturwissenschaften, 96(9), 1011–1025. https://doi.org/10.1007/s00114-009-0560-7
Nixdorf-Bergweiler, B. E., & Bischof, H.-J. (2007). A stereotaxic atlas of the brain of the zebra finch, Taeniopygia guttata. .
Odum, E. P. (1942). A Comparison of Two Chickadee Seasons. Bird-Banding, 13(4), 154–159. https://doi.org/10.2307/4509755
Patel, S. N., Clayton, N. S., & Krebs, J. R. (1997). Spatial learning induces neurogenesis in the avian brain. Behavioural Brain Research, 89(1/2), 115–128. https://doi.org/10.1016/S0166-4328(97)00051-X
Pravosudov, V. V. (2006). On seasonality in food-storing behaviour in parids: Do we know the whole story? Animal Behaviour, 71(6), 1455–1460. https://doi.org/10.1016/j.anbehav.2006.01.006
Pravosudov, V. V., & Omanska, A. (2005). Prolonged moderate elevation of corticosterone does not affect hippocampal anatomy or cell proliferation rates in mountain chickadees (Poecile gambeli). Journal of Neurobiology, 62(1), 82–91. https://doi.org/10.1002/neu.20069
Pravosudov, V. V., & Smulders, T. V. (2010). Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1542), 859–867. Scopus. https://doi.org/10.1098/rstb.2009.0216
Pravosudov, V. V., Lavenex, P., & Clayton, N. S. (2002). Changes in spatial memory mediated by experimental variation in food supply do not affect hippocampal anatomy in mountain chickadees (Poecile gambeli). Journal of Neurobiology, 51(2), 142–148. https://doi.org/10.1002/neu.10045
Pravosudov, V. V., Kitaysky, A. S., & Omanska, A. (2006). The relationship between migratory behaviour, memory and the hippocampus: An intraspecific comparison. Proceedings of the Royal Society B: Biological Sciences, 273(1601), 2641–2649. https://doi.org/10.1098/rspb.2006.3624
Pytte, C. L. (2016). Adult neurogenesis in the songbird: Region-specific contributions of new neurons to behavioral plasticity and stability. Brain, Behavior and Evolution, 87(3), 191–204. https://doi.org/10.1159/000447048
Pytte, C. L., Parent, C., Wildstein, S., Varghese, C., & Oberlander, S. (2010). Deafening decreases neuronal incorporation in the zebra finch caudomedial nidopallium (NCM). Behavioural Brain Research, 211(2), 141–147. Scopus. https://doi.org/10.1016/j.bbr.2010.03.029
Roach, S. P., Lockyer, A. C., Yousef, T., Mennill, D. J., & Phillmore, L. S. (2016). Vocal production and playback of altered song do not affect ZENK expression in black-capped chickadees (Poecile atricapillus). Behavioural Brain Research, 298, 91–99. https://doi.org/10.1016/j.bbr.2015.10.047
Roth, T. C., II, & Pravosudov, V. V. (2009). Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: A large-scale comparison. Proceedings of the Royal Society B: Biological Sciences, 276(1656), 401–405. https://doi.org/10.1098/rspb.2008.1184
Roth, T. C., Brodin, A., Smulders, T. V., LaDage, L. D., & Pravosudov, V. V. (2010). Is bigger always better? A critical appraisal of the use of volumetric analysis in the study of the hippocampus. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1542), 915–931. https://doi.org/10.1098/rstb.2009.0208
Roth, T. C., LaDage, L. D., & Pravosudov, V. V. (2011). Variation in hippocampal morphology along an environmental gradient: Controlling for the effects of day length. Proceedings of the Royal Society B: Biological Sciences, 278(1718), 2662–2667. https://doi.org/10.1098/rspb.2010.2585
Roth, T. C., LaDage, L. D., Freas, C. A., & Pravosudov, V. V. (2012). Variation in memory and the hippocampus across populations from different climates: A common garden approach. Proceedings of the Royal Society B: Biological Sciences, 279(1727), 402–410. https://doi.org/10.1098/rspb.2011.1020
Roth, T. C., Stocker, K., & Mauck, R. (2017). Morphological changes in hippocampal cytoarchitecture as a function of spatial treatment in birds. Developmental Neurobiology, 77(1), 93–101. https://doi.org/10.1002/dneu.22413
Sherry, D. F., & Guigueno, M. F. (2019). Cognition and the brain of brood parasitic cowbirds. Integrative Zoology, 14(2), 145–157. https://doi.org/10.1111/1749-4877.12312
Sherry, D. F., & Hoshooley, J. S. (2007). Neurobiology of spatial behavior. In: Ecology and behavior of chickadees and titmice: An integrated approach. https://doi.org/10.1093/acprof:oso/9780198569992.003.0002
Sherry, D. F., & MacDougall-Shackleton, S. A. (2015). Seasonal change in the avian hippocampus. Frontiers in Neuroendocrinology, 37, 158–167. https://doi.org/10.1016/j.yfrne.2014.11.008
Sherry, D. F., Jacobs, L. F., & Gaulin, S. J. (1992). Spatial memory and adaptive specialization of the hippocampus. Trends in Neurosciences, 15(8), 298–303. https://doi.org/10.1016/0166-2236(92)90080-r
Shiflett, M. W., Tomaszycki, M. L., Rankin, A. Z., & DeVoogd, T. J. (2004). Long-term memory for spatial locations in a food-storing bird (Poecile atricapilla) requires activation of NMDA receptors in the hippocampal formation during learning. Behavioral Neuroscience, 118(1), 121–130. https://doi.org/10.1037/0735-7044.118.1.121
Smulders, T. V. (2017). The avian hippocampal formation and the stress response. Brain, Behavior and Evolution, 90(1), 81–91. https://doi.org/10.1159/000477654
Smulders, T. V. (2021). Telencephalic regulation of the HPA axis in birds. Neurobiology of Stress, 15, 100351. https://doi.org/10.1016/j.ynstr.2021.100351
Smulders, T. V., Sasson, A. D., & DeVoogd, T. J. (1995). Seasonal variation in hippocampal volume in a food-storing bird, the black-capped chickadee. Journal of Neurobiology, 27(1), 15–25. https://doi.org/10.1002/neu.480270103
Smulders, T. V., Casto, J. M., Nolan, V., Ketterson, E. D., & DeVoogd, T. J. (2000). Effects of captivity and testosterone on the volumes of four brain regions in the dark-eyed junco (Junco hyemalis). Journal of Neurobiology, 43(3), 244–253.
Stankiewicz, A. M., Swiergiel, A. H., & Lisowski, P. (2013). Epigenetics of stress adaptations in the brain. Brain Research Bulletin, 98, 76–92. https://doi.org/10.1016/j.brainresbull.2013.07.003
Sweatt, J. D. (2016). Neural plasticity and behavior – sixty years of conceptual advances. Journal of Neurochemistry, 139(S2), 179–199. https://doi.org/10.1111/jnc.13580
Tarr, B. A., Rabinowitz, J. S., Imtiaz, M. A., & DeVoogd, T. J. (2009). Captivity reduces hippocampal volume but not survival of new cells in a food-storing bird. Developmental Neurobiology, 69(14), 972–981. https://doi.org/10.1002/dneu.20736
Thompson, C. K., Meitzen, J., Replogle, K., Drnevich, J., Lent, K. L., Wissman, A. M., Farin, F. M., Bammler, T. K., Beyer, R. P., Clayton, D. F., Perkel, D. J., & Brenowitz, E. A. (2012). Seasonal changes in patterns of gene expression in avian song control brain regions. PLOS ONE, 7(4), e35119. https://doi.org/10.1371/journal.pone.0035119
Tramontin, A. D., & Brenowitz, E. A. (2000). Seasonal plasticity in the adult brain. Trends in Neurosciences, 23(6), 251–258. https://doi.org/10.1016/s0166-2236(00)01558-7
Tramontin, A. D., Smith, G. T., Breuner, C. W., & Brenowitz, E. A. (1998). Seasonal plasticity and sexual dimorphism in the avian song control system: Stereological measurement of neuron density and number. Journal of Comparative Neurology, 396(2), 186–192. https://doi.org/10.1002/(SICI)1096-9861(19980629)396:2<186::AID-CNE4>3.0.CO;2-X
van Praag, H. (2008). Neurogenesis and Exercise: Past and Future Directions. NeuroMolecular Medicine, 10(2), 128–140. https://doi.org/10.1007/s12017-008-8028-z
van Praag, H., Kempermann, G., & Gage, F. H. (2000). Neural consequences of environmental enrichment. Nature Reviews. Neuroscience, 1(3), 191–198. https://doi.org/10.1038/35044558
Wada, H., Newman, A. E. M., Hall, Z. J., Soma, K. K., & MacDougall-Shackleton, S. A. (2014). Effects of corticosterone and DHEA on doublecortin immunoreactivity in the song control system and hippocampus of adult song sparrows. Developmental Neurobiology, 74(1), 52–62. https://doi.org/10.1002/dneu.22132
Zhao, C., Deng, W., & Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell, 132(4), 645–660. https://doi.org/10.1016/j.cell.2008.01.033
Funding
This work was supported by a Discovery Grant from Natural Sciences and Engineering Research Council awarded to LSP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose.
Ethics approval
All capturing and experimental procedures were approved by both the Dalhousie University Committee on Laboratory Animals (Permit #12-092) and the Canadian Wildlife Service (#ST2779).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phillmore, L.S., Aitken, S.D.T. & Parks, B.M.B. Understanding hippocampal neural plasticity in captivity: Unique contributions of spatial specialists. Learn Behav 50, 55–70 (2022). https://doi.org/10.3758/s13420-021-00504-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-021-00504-2