Abstract
We propose an expansion of neuroecological comparisons to include the capabilities of brainless and non-neural organisms. We begin this enterprise by conducting a systematic search for studies on learning in echinoderms. Echinodermata are marine invertebrates comprising starfish, brittle stars, sea cucumbers, sea urchins, and sea lilies. Animals in this phylum lack any centralized brain and instead possess diffuse neural networks known as nerve nets. The learning abilities of these animals are of particular interest as, within the bilaterian clade, they are close evolutionary neighbors to chordates, a phylum whose members exhibit complex feats in learning and contain highly specialized brains. The learning capacities and limitations of echinoderms can inform the evolution of nervous systems and learning in Bilateria. We find evidence of both non-associative and associative learning (in the form of classical conditioning) in echinoderms, which was primarily focused on starfish. Additional evidence of learning is documented in brittle stars, sand dollars, and sea urchins. We then discuss the evolutionary significance of learning capabilities without a brain, the presence of embodied cognition across multiple groups, and compare the learning present in echinoderms with the impressive cognitive abilities documented in the oldest linage group within vertebrates (the major group within the phylum of chordates), fish.
Similar content being viewed by others
"Echinoderms have been called the strangest animals on Earth … Starfish, sea urchins, brittle stars, sea cucumbers, and sea lilies … are built like nothing else on the planet.” (P. Holland, 2011, p.70)
Introduction
The phylum of Echinodermata, though falling into the giant clade of bilaterian animals, is better known for its five-fold symmetry in its adult form (Fig. 1). But the clade also makes an interesting case for neuroecological considerations (Sherry, 2006). Sherry (2006) called neuroecology “the study of adaptive variation in cognition and the brain” (p. 167). Echinoderms force an expansion of that description from “brain” to “nervous system” because, while they possess a nervous system, which we describe later, they lack any centrally cephalized group of neurons that could be called a brain. And yet phylogenetically, the clade is closely related to another phylum with some spectacular brains, that of Chordata (Fig. 2). Examining the cognitive capacities of echinoderms and comparing them with Chordata thus make an intriguing enterprise. We start this comparison by examining the extant literature on learning in echinoderms, placing the findings in a neuroecological context.
Echinoderm animals. a The starfish, Marthasterias glacialis. b The brittle star, Ophioderma wahlbergii. c The feather star, Himerometra robustipinna. d The sea urchin, Sphaerechinus granularis. e The sea cucumber, Stichopus herrmann. In color online. Sources: (a) https://commons.wikimedia.org/wiki/File:Estrella_espinosa_com%C3%BAn_(Marthasterias_glacialis),_Madeira,_Portugal,_2019-05-31,_DD_57.jpg Author: Dioego Delso. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en. (b) https://commons.wikimedia.org/wiki/File:Serpent_skinned_brittlestar_at_Partridge_Point_P7190590.JPG Author: Peter Southwood. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en. (c) https://commons.wikimedia.org/wiki/File:Colobometridae_-_Cenometra_bella.jpg Author: Hectonichus. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en. (d) https://commons.wikimedia.org/wiki/File:Erizo_de_mar_viol%C3%A1ceo_(Sphaerechinus_granularis),_Madeira,_Portugal,_2019-05-31,_DD_36.jpg Author: Dioego Delso. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en. (e) https://commons.wikimedia.org/wiki/File:Sea_Cucumber_(Stichopus_herrmanni)_(8456845410).jpg Author: Bernard Dupont. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en
To examine the evolutionary origins of any behavior, cognitive trait, element in the nervous system, or indeed of any trait at all, scientists must compare species or species groups, such as families or orders. Neuroecology, with its focus on adaptive variations and their relationships with underlying neural structures, must adopt such a comparative framework as well. Different modes or approaches to comparing could be carried out to answer different types of questions (Cheng, 2016; Sherry, 2006; Shettleworth, 2010).
One way is to take a sizeable sample of phylogenetically independent units and examine if some characteristic is correlated with some neural or cognitive trait or both. This examined characteristic, which could characterize lifestyle, behavior, or the physical or biotic environment, could be considered a contributing factor to the neural or cognitive trait should a significant correlation be found. Phylogenetic independence of the groups being compared is crucial for this correlational exercise, for the same reason that independent sampling from a target population is important for experiments of any kind. Related animals typically show similarities in their traits based on shared phylogenetic history, and shared history is what this mode of the comparative method aims to control for by picking phylogenetically independent units. This independent-units approach has featured in some of David Sherry’s neuroecological work, notably the comparisons that showed that food-storing passerines possess relatively larger hippocampus sizes than non-storing passerines (Krebs et al., 1989; Sherry et al., 1989).
Another mode could be considered as a variant of the independent-units analysis but is different enough to require a description. That is to compare closely related pairs (or n-tuples) of species that differ in some lifestyle or habitat characteristic, akin to a matched-pairs experiment in experimental work as opposed to the random assignment of subjects. The difference is that in the comparative method, nature is doing the assignment rather than experimenters. This method amounts to controlling for phylogenetic history by matching most of the phylogeny of pairs of species. In the food-storing theme again, the comparison of closely related corvids and parids that store food to different extents exemplifies this approach (Hampton et al., 1995; Olson et al., 1995).
A third mode of the comparative method is used to trace the origins of behavior. For this method, a reliable and agreed-upon phylogenetic map of the clade in which one is interested must be available. Looking at units on the map that do and do not possess a trait of interest (including neural or cognitive traits) could lead one to inferences about when and perhaps how often that trait arose in evolutionary history (Perry et al., 2013). The phylogeny is important because if the map does not represent the terrain, the X on the map cannot be used to make inferences. The mapping out of the phylogeny of a trait can often lead to insights about factors that drove the evolution of that trait (e.g., Ord et al., 2015). An example of this line of argument related to our current article is that nervous systems have been argued to have arisen twice because they are found in distantly related groups of animals, the ctenophores (comb jellies) on one side, and the Cnidaria (jellyfish, box jellyfish, hydra, corals, sea anemones; see Cheng, 2021) plus the massive group of phyla known as bilaterians on the other side, but not in two other phyla: Porifera (sponges), and Placozoa (which has no common name) (Moroz, 2015; Moroz & Kohn, 2016).
Taking this third mode of comparison, traits in echinoderms inform us about the evolutionary origins of traits in the much-studied clade of Chordata (Fig. 2). That is because the two phyla are neighbors on the bilaterian phylogenetic tree. If a trait is found to be widespread in both echinoderms and chordates, the evolutionary inference would be that such a trait evolved in a common ancestor to both phyla, or even earlier. With this perspective in mind, we examine phenomena of basic learning in echinoderms. By basic learning, we mean associative learning (classical and operant conditioning) and non-associative learning, habituation and sensitization. In the course of reviewing the literature, we discovered phenomena indicative of memory, but hard to classify in the scheme of associative vs. non-associative learning, which we also report. In such an enterprise, it is important to map out which groups (species, genera, or higher-level grouping) have not been shown to possess (despite rigorous testing) a trait as well as which groups do (Perry et al., 2013). A comprehensive map marked with “yes” and “no” labels is needed for inferences about evolutionary origins (see Ord et al., 2015’s Fig. 3 for a detailed example in lizards).
a Diagram of the water vascular system in the starfish. b Image of the tube feet projections (podia) in the starfish, Pycnopodia helianthoides. c Diagram of the decentralized nervous system in the starfish featuring the circular nerve ring and the five radial nerves. In color online.
Sources: (a) Modified from an original public domain image: https://commons.wikimedia.org/wiki/File:FMIB_52615_Diagram_of_water-vascular_system_of_a_starfish_;.jpeg Author: Augusta Foote Arnold. License: https://creativecommons.org/licenses/by-sa/2.0 /deed.en. (b) https://commons.wikimedia.org/wiki/File:Pycnopodiahelianthoides-tubefeet.jpg Author: Stickpen. License: https://creativecommons.org/licenses/by-sa/2.0/deed.en. (c) Modified from an original public domain image: https://commons.wikimedia.org/wiki/File:NSRW_Starfish.png. Author: The New Student's Reference Work (1914) edited by Chandler B. Beach and Frank Morton McMurry. License: https://creativecommons.org/licenses/by-sa/2.0 /deed.en
In the rest of this paper, we first provide background on echinoderms, including their major classes, their body plans, their lifestyle, and their nervous system organization. Then we present a systematic but non-exhaustive review of learning in echinoderms based on a literature search. Based on this literature, we discuss the origins of learning in chordates, in animals in general, and even beyond animals.
Echinodermata background
Echinodermata is a phylum of marine invertebrates, with a worldwide distribution of roughly 7000 extant species. Echinoderms are most easily identified as adults via their five-fold symmetry, yet this group exhibits bilateral symmetry during their larval life stages. Classes in the phylum comprise starfish (or sea stars, class Asteroidea), brittle stars (Ophiuroidea), sea cucumbers (Holothuroidea), sea urchins (Echinoidea), and sea lilies (Crinoidea; Fig. 1; P. Holland, 2011). A sixth group, the sea daisies, with three species in the genus Xyloplax known to date, had been considered a separate class, but more recent molecular phylogenetic work places them within Asteroidea (Linchangco Jr. et al., 2017). Living member species are benthic organisms, either remaining stationary or moving slowly along the sea floor across a wide range of ocean depths, from the intertidal shallows to the deep-sea floor (Hyman, 1955; Willows & Corning, 1975). In spite of this general pattern in lifestyle, some species of sea lilies (Crinoidea) are capable of swimming through coordinated movements of their arms using contractible muscle tissues (Birenheide & Motokawa, 1996; Grimmer & Holland, 1987; Hyman, 1955) while some deep-sea cucumbers (Holothuroidea) showcase limited swimming abilities via muscular contraction as an anti-predatory response (Miller & Pawson, 1990). Echinoderms serve as an interesting model for comparative study across a range of biological fields, including the evolutionary history of cognition, given their close phylogenetic distance to vertebrates (Chordata) within the deuterostome clade compared to other invertebrate lineages (Fig. 2; Cameron et al., 2000; Pawson, 2007).
Anatomically, echinoderms are characterized by pentamerous radial symmetry in the adult form of most species. Even the classes without the obvious five arms contain five-fold symmetry when examined closely (P. Holland, 2011). The sea urchins (Echinoidea), bristling with spines (Fig. 1d), and the sea cucumbers, resembling long blobs or worms (Fig. 1e), contain five zones in which tube feet are arranged. Echinoderms possess a unique circulatory system relying on hydraulic pressure. This water vascular system (Fig. 3a) is utilized for respiration, nutrition and waste transport, sensation, as well as locomotion via podia or “tube feet” that are tubular projections arranged along the animal’s oral surface (Fig. 3b; McCurley & Kier, 1995). These tube feet are controlled via the water vascular system; incoming sea water passes through the central ring canal and down radial canals in each arm (Fig. 3a). Along each radial canal, connected via lateral canals, are water reservoirs called “ampulla” that allow for the extension and retraction of each individual external tube foot (Fig. 3a). Locomotion via these feet requires a coordinated choreography or stepping with tube feet extending in a chosen direction across all five arms (Fig. 3b). These extensions and retractions are coupled with adhesive/de-adhesive secretions from glands within each foot allowing for locomotion along a variety of surfaces (Flammang, 1996; Kerkut, 1954). Tube-feet-powered locomotion is present across the phylum. While most commonly studied in starfish, both sea cucumbers and sea urchins also move through tube feet stepping, though the former group can also move through rhythmic muscular contraction while the latter are also thought to simultaneously use the spines on their oral surface to move (Domenici et al., 2003). In contrast, brittle stars (Ophiuroidea) primarily achieve locomotion by relying on coordinated oscillations of their arms to travel, while their tube feet are used to grip substrates (Astley, 2012; P. Holland, 2011; Smith, 1937).
Echinoderm lifestyles and directed behaviors
Across the phylum, echinoderms show a variety of behavioral strategies tied to directed locomotion during both foraging and anti-predator behaviors. In their larval form, echinoderms are typically planktotrophic passive suspension feeders, collecting and transporting suspended particles to the oral opening via a ciliated band that surrounds this structure (Strathmann, 1971). In their adult forms, some members such as sea lilies are sessile suspension feeders passively filtering food particles out of the water stream using fan-like arms (Rutman & Fishelson, 1969), though some sea lilies can exhibit short bursts of movement, either swimming or crawling to find better foraging sites or as anti-predation behaviors (Janevski & Baumiller, 2010). Other Echinodermata members are herbivore grazers, slowly moving along substrates feeding on algae (sea urchins) or deposit feeders burrowing into the seafloor (sea cucumbers) ingesting plant and animal materials (Andrew & Underwood, 1993; Lawrence & Sammarco, 1982). Starfish and brittle stars show a variety of foraging behaviors and can be active olfactory predators, targeting reef coral, mollusks, gastropods, or other echinoderms as well as burrowing scavengers or deposit feeders. Brittle stars are more likely to pick up little pieces of food, while starfish actively hunt bivalves (Beddingfield & McClintock, 1993; Güler & Lök, 2015; P. Holland, 2011). Starfish use their tube feet to prise open bivalve shells. Just a tiny opening allows these “voracious predators” (P. Holland, 2011, p, 73) to start everting their stomach with its digestive juices and break down proteins, thus allowing the hunter to prise open the shells further. Still others, such as basket stars (Ophiuroidea) are sessile suspension feeders (Emson et al., 1991).
Coordinated behavior and the decentralized brain
Despite numerous examples of coordinated motor functions, directed locomotion, and learning behaviors, the nervous system in the phylum Echinodermata is rather unsophisticated, lacking cephalization or a central executive center (brain). Given that many echinoderms are active foragers, and must both move and handle food in a directed manner (McClintock & Lawrence, 1981, 1985), how are these behaviors accomplished in animals without a brain?
Though the nervous system in echinoderms lacks a centralized brain structure (Pentreath & Cobb, 1972), nerve groups distributed across the animal’s body are able to become temporary coordinating centers for directed behaviors (Kerkut, 1954; Willows & Corning, 1975). In a key commonality with vertebrate neuroanatomy, all member classes of Echinodermata possess sufficient specialization of the neural tissues to constitute a central nervous system, CNS (Mashanov et al., 2009), as well as the presence of non-neural cells similar to glial cells. These glial cells are critical to the development of specialized neural tissues (Mashanov et al., 2009; Pinto & Gotz, 2007) as well as the regeneration of the neural tissue after limb loss in echinoderms (Mashanov et al., 2008).
The echinoderm CNS is composed of both a central nerve ring (circumoral nerve ring) situated around the oral opening and (typically five) radial nerve cords that branch from the central ring and extend out to each arm’s muscular tissue (Fig. 3c; Cobb, 1995; Mashanov et al., 2016). The echinoderm neural system, except for the class Crinoidea (sea lilies), contains two subsystems once believed to be distinctly separate but are now thought to be extensively interconnected (Hoekstra et al., 2012), the ectoneural and hyponeural systems (Hyman, 1955; Mashanov et al., 2006). The ectoneural system comprises the central nerve ring and outer radial nerves and has motor and sensory functions while the hyponeural subsystem is the inner layer of the radial nerve cords and is involved in locomotive motor functions (Cobb, 1987). Early research in echinoderms theorized that the central nerve ring structure acted as a brain-like control center for coordinated movement (Kerkut, 1955). Now, however, the central nerve ring is thought to share information between the radial nerve cords, while the radial nerves coordinate movement (Cobb, 1995). Studies on the control of movement furnish the evidence.
Directed movement with a pentaradial layout requires one appendage to become the leader arm while the four other arms cooperate via the stepping of their tube feet extensions in the lead-arm direction or by coordinated rowing behaviors in species of Ophiuroidea (Astley, 2012). Both starfish and brittle stars can exhibit coordinated behaviors during directed locomotion under decentralized neural control, which can be advantageous, as this design makes the system resilient to injury and able to modify behavior after damage. After arm removal (one or more) in both starfish and brittle stars, these animals are still able to execute synchronized directed movements using their remaining appendages (Arshavskii et al., 1976; Clark et al., 2019; Matsuzaka et al., 2017; Piscopo et al., 2005). If the central nerve ring is lesioned at two sites, the arms beyond these lesions cease to cooperate with the lead arm (Clark et al., 2019; Kerkut, 1954). However, if a singular lesion is made to the central ring, coordination between arms persists (Clark et al., 2019; Kerkut, 1954). This lesioning work suggests that the central nerve ring does not itself act as the coordinating center for intra-arm behaviors, but instead acts to connect and transfer information bidirectionally between the radial nerves in each arm (Arshavskii et al., 1976; Clark et al., 2019; Cobb, 1987). Lesions of the radial nerve in isolated starfish arms suggest that the control centers for locomotion may be within the junction of the radial nerve and the central nerve ring, with these sites across the animal’s body acting as a decentralized brain for coordinated behaviors (Kerkut, 1954; Willows & Corning, 1975). In one accepted sense of the term then, such control within the arms exhibits embodied cognition, a phenomenon in which cognitive processes are distributed to areas outside of the brain (for review see Cheng, 2018). This type of cognitive control within the animal’s limbs themselves is sometimes also found in animals with brains, the octopus being a prime example (Cheng, 2018; Hochner, 2012). In spite of this lack of a central brain structure and the use of temporary coordinating centers across the animal’s body, multiple species of echinoderms show ample evidence of associative learning and memory.
Learning in echinoderms
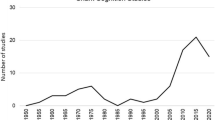
We began our search for literature on the database Web of Science (Clarivate) in a similar fashion to procedures followed by Cheng (2021). Terms for organisms were crossed with terms on learning as topic terms using the logical operator AND in Web of Science. Organism names included: echinoderm, starfish, sea star, brittle star, sea urchin, sea cucumber, sand dollar, crinoid; while learning terms included habituat*, sensitiz*, sensitis*, conditioning, and learning.
Additionally, we enlisted the Macquarie University Library support services to expand our literature search using the BIOSIS citation index (Clarivate), Web of Science (Clarivate) and Natural Science Collection (ProQuest) databases. These searches used organism terms (echinoderm*, sea star*, brittle star*, sea urchin*, sea cucumber*, sand dollar*, crinoid* or sea lil*) crossed with terms connected to cognition and learning (learn*, associative learning, non-associative learning, learning capabilit*, habituat*, sensiti?ation, facilitation, classical conditioning, Pavlovian conditioning, or operant conditioning). Output lists were searched for articles on learning and reference trails to earlier literature were followed. The list produced a smattering of evidence for non-associative learning and a variety of studies investigating associative learning.
Early in the 20th century, H. S. Jennings studied the starfish Asterias forreri de Loriol, found on the west coast of California, in detail (Jennings, 1907). The study lacked any graphs of data but provided rich descriptions of behavior, with many behaviors suggestive of both non-associative and associative learning.
Defensive behaviors in structures surrounding the starfish’s gills suggest a sensitization process (Jennings, 1907) akin to the much-studied sensitization of the stinger release from nematocysts (stinging cells) in sea anemones, which are cnidarians with nerve nets as nervous systems (review: Cheng, 2021). Around the gills of starfish are round clumps called rosettes containing structures called pedicellariae (singular: pedicellaria), which can open up in what Jennings described as something like a “hundred-jawed monster” (p. 61), each ready to pinch in attack. The rosettes rise up from their normal state in preparation for pinching by pedicellariae, whose function is to hold on to something, a prey or a predator. Paralleling sea anemones (Pantin, 1935), it generally takes more than a single mechanical stimulus to elicit the rising of rosettes (Jennings, 1907). Various chemicals, however, may prepare the rosettes, so that they would rise with even light mechanical stimulation. The sensitization could spread to neighboring rosettes, or even to rosettes on other arms, suggesting to Jennings some transmission across the nerve net of starfish.
A phenomenon suggestive of habituation was also described by Jennings (1907). The onset of a moderate level of light typically disrupts ongoing activities such as eating, but after 20–45 min, the starfish typically resumes activities again, suggestive of habituation. No systematic controls of any kind were described, so that this interpretation is far from certain. In more recent work, evidence of habituation has expanded beyond Asteroidea to other echinoderm classes. Roy et al. (2012) characterized the behavioral responses to water turbulence in sea urchin larvae (Strongylocentrotus droebachiensis). In attempting to disperse away from parental waters, these larvae will actively travel to the surface under calm conditions, but water turbulence disrupts this directed behavior. After a previous exposure to low levels of water turbulence, larvae were more directed toward the surface on the second trial, suggesting that individuals habituated to the turbulence. Additional evidence of habituation has emerged recently in the sea cucumber, one of a few underrepresented echinoderm classes with regards to cognition. Hamel et al., (2021) reported anticipatory immune and hormonal responses in the orange-footed sea cucumber (Cucumaria frondosa). If individuals were chronically exposed to a chemical indicator of a predator (predator’s scent or injured conspecifics) for 3 days without an attack, the immune response would return to baseline, suggesting habituation to these cues.
Three phenomena suggestive of associative learning are worth describing (Jennings, 1907). Jennings (1907) described one starfish struggling to predate on a prickly sea urchin. The sea urchin, also an echinoderm, launched its pedicellariae in defense and there ensued a battle of pedicellariae, described as a “spirited combat” (p. 88) with the starfish always being the aggressor. With much damage to pedicellariae after 5 min or so, however, the starfish “had had enough” (p. 88) and began withdrawing from combat. Five min after the battlers had separated, the sea urchin was placed in contact with the starfish once again. This time, the pedicellariae of the starfish rose in defense, but the starfish did not attempt to capture the sea urchin, suggestive of a form of avoidance learning. Similar behaviors have been reported in the foraging choices of the star fish Leptasterias polaris (Mercier & Hamel, 2008). Here, researchers were focused on characterizing the symbiotic relationship between a number of gastropod species and the epibiont sea anemone Allantactis parasitica. This relationship reduced the gastropod’s predation risk, with researchers reporting the auxiliary finding that predatory L. polaris learned to avoid foraging upon gastropod patches with this sea anemone after a previous unsuccessful attack.
Movement of starfish furnished another suggestion of associative learning (Jennings, 1907), a form of directional memory, as it was called a century later (Yoshimura et al., 2012; Yoshimura et al., 2018). A starfish that moves in a particular direction typically heads off again with the same leading arm after an interruption. The memory is egocentric in that it is the same arm that leads no matter how the starfish has been placed and regardless of the allocentric direction in which the starfish moves. Another form of directional memory is shown when one (or more) arms comes into contact with something. After displacement, those arms formerly in contact typically lead off. Jennings suggested that such a phenomenon, if found in a “higher animal”, would be considered as a display of memory. Yoshimura and colleagues’ work (Yoshimura et al., 2012, 2018), described in more detail later, amply confirm Jennings’ observations in a species of sea urchins, but these 21st-century works did not cite Jennings (1907).
The most detailed investigations that Jennings (1907) carried out were on the righting reaction. This is what a starfish does after it is placed with its aboral (dorsal) side down; the starfish tries to turn itself back right side up again (Fig. 4; Ji et al., 2012). Among other things, righting functions to avoid damage to sensitive structures such as gills on the aboral side. To right itself, one or two arms needs to bend and attach some of the oral (ventral) side of its tube feet to the substrate. Then the rest of the starfish needs to flip over. Some coordination between arms is needed (Fig. 4). If all arms attach tube feet to the substrate, the starfish stays upside down in a tug-of-war with itself. Jennings noted that starfish had preferred arms for righting themselves.
The starfish righting behavior explored in Jennings (1907). Typically, the oral surface of these animals faces downward on the substrate they are clinging to. When overturned (1), these animals perform a righting behavior via the coordinated use of their tube feet and arm movements to somersault, returning them to their normal position. This particular sequence begins with all arms extending upward (2), then two adjacent arms bend and attach to the ground forming the pivot point (3). The other arms lift and tilt the animal’s body vertically until the animal turns over (4–6). Image sourced from Ji et al., 2012. License: https://creativecommons.org/licenses/by/4.0/
In the spirit of detailed single-animal operant work, Jennings tried to train starfish to use non-preferred arms to right themselves. The most thorough investigations were on just two starfish. In reinforcement-learning terms, positive punishment was used to change behavior. Attempts to use preferred arms, or any other arms than the non-preferred pair of arms that Jennings decided on in advance, were punished by prodding with a glass rod. Ten righting trials, called “lessons”, were given each day over multiple days. The training produced limited success, with unpunished tests showing some use of the trained non-preferred arms as leading arms in righting. With few such test trials and a lack of inferential statistics, however, the import of the training studies is far from clear.
Jennings’ (1907) work produced many interesting observations suggestive of learning in starfish. A dearth in the tabulation of data and a total lack of inferential statistics, however, made it hard to be fully convinced that starfish learn. Later studies on learning in echinoderms in the 20th century mostly featured associative learning. Classical conditioning, phenomena of memory, and inhibitory processes have been investigated in echinoderms. One intriguing piece of evidence on sensitization, however, came to light.
A form of sensitization was found in the allergic reaction of the sand dollar Mellita quinquisperforata to human serum (Smith & Smith, 1985). These sand dollars exhibit two kinds of allergic reactions to human serum: the release of red granules, thought to be protective, and the release of histamine. With a second dose of human serum, the release of red granules increased, showing sensitization. Sensitization was not found for the release of histamine. In fact, histamine release decreased with the second dose of human serum. This phenomenon seems to reflect learning in the immune system rather than the nervous system.
Classical conditioning in echinoderms has been shown in multiple studies, mostly on starfish. The Pacific starfish, Pisaster giganteus, was trained to associate 15 min of light presentation with food; the food was presented simultaneously with the light (Landenberger, 1966). Food was placed on the bottom of the test aquarium and the starfish had to learn to go to the bottom, on which they did not dwell at other times. Trials were well spaced for conditioning experiments, with the intertrial interval at 48 h. The animals learned in 8 trials to go to the bottom for food in the presence of light. Subsequent control conditions were run. With light only along with the absence of food, or with light and food presentations separated by 12 h, the conditioned response disappeared. The separate presentations of light and food provided an excellent control for non-associative learning processes.
Food conditioning was shown in another starfish, Luidia clathrata (McClintock & Lawrence, 1982). The conditioned stimulus again revolved around light level, either 15 min of light each day with the rest of the time being dark or its reverse, 15 min of darkness in a lit environment. Activity level was measured as the conditioned response, and activity levels increased during the conditioned stimulus over the course of 30 days of training. Extinction procedures were then instituted, with the conditioned stimulus no longer associated with food. The conditioned response waned. No explicitly unpaired condition was run.
Other studies used punishment procedures to show associative learning in echinoderms. Classic work by Diebschlag (1938) featured shocks delivered from a pedicellaria-battery device, although how such a device worked was not described. Starfish, brittle stars, and some sea urchins were shocked for entering certain areas of an arena. Most of the work was on various species of starfish and brittle stars as sea urchins proved hard to work with in this context. Discrimination learning was also at play in these forms of avoidance learning, as the arena was divided into areas with different textures (e.g., rough vs. smooth, wavy vs. smooth), different light levels, or sometimes with tactile and light-level discriminanda in combination. Among brief descriptions of some failures of the training procedures to produce learning were documented successful learning on the part of the echinoderms. For example, starfish and brittle stars preferred rough texture over smooth, and would inevitably settle on rough texture. After being disturbed, so that the animals started moving, Diebschlag then punished them with shock whenever they touched the rough texture. Shock would cause the test subjects to withdraw the arm that encountered the shock. After a number of shocks, Diebschlag reported that both starfish and brittle stars would withdraw their arm as soon as it touched the rough texture, before shock was applied manually by the experimenter. Animals would even withdraw other arms that had not been punished before, leading Diebschlag to conclude that the animal as a whole learned, not individual arms. Some animals even settled on the smooth texture, a behavior never observed before the punishment procedures were instituted. The learning did not last long, with the animals either forgetting or exploring again after 10–30 min, but often, a form of savings was reported. The animals relearned the conditioned-stimulus-shock contingency quickly. Diebschlag gave plenty of individual records, but more systematic reporting of all animals and all phases of experiments would have enhanced the presentation—no records of the saving phenomenon, for example, were presented. In a study originally reported in Russian, evidence of tactile conditioning in the starfish (Asterias rubens) has also been documented using food reinforcement (Sokolov, 1961, described in Willows & Corning, 1975) with the suggestion that this conditioned preference is retained for up to three months.
Shock procedures continued in the second half of the 20th century. The starfish Marthasterias glacialis was shocked for touching food in one study (Valentinčič, 1978, 1980). Cotton soaked with food (L-cysteine) was wrapped around a wire through which shock was delivered when the common starfish touched it with their tube feet. Results suggest some avoidance learning but the work was beset with problems, lacking any inferential statistics, a definition of what a trial was, and any consideration of the duration for which starfish refrained from touching the proffered food. A better reported study with inferential statistics examined the same species’ reactions to various chemicals, some of which elicited arm movements (Valentinčič, 1985). Mild shock paired with the presentation of L-cysteine or L-proline reduced the arm movement normally elicited by these chemicals, while other kinds of chemicals still elicited arm movements. This study suggested inhibitory processes in learning in starfish.
Inhibition of natural, unconditioned responses was the featured theme in another study, on the common starfish Asterias rubens (Shulgina, 2006). When placed at the bottom of a small aquarium, these starfish would rise to the top, clinging to a wall at the top of the water level; they would do this repeatedly. Shulgina (2006) then punished the animals for rising to the top in one of two ways: either a squirt of fresh water was directed at them (desalting) or they were tapped with a pipette (which was used for the desalting manipulation). The starfish would descend to the bottom, and then rose again after a short while. With repeated punishment, the starfish stayed at the bottom for longer and longer, for hours or, in one case, for two days. Shulgina (2006) interpreted the findings as showing active inhibition, also called “forbidden” inhibition in the paper.
Various other observations and manipulations suggested to Shulgina (2006) that inhibition of natural behavior was at play. When at first staying on the bottom, Shulgina (2006) noted that the tube feet of the starfish were clamped to the substrate, with the echinoderm staying in one place. Eventually, the starfish could move about on the bottom. Holding on with the tube feet was interpreted as a form of inhibiting the behavior of rising to the top of the water. Forms of disinhibition were also found, further supporting the interpretation of inhibition and providing evidence that the starfish were not simply too fatigued to rise again. While the starfish were at the bottom, tapping them or squirting fresh water at them would make them rise to the top again. In fact, simply shaking the water was enough to induce the starfish to rise up again. While the study furnished a rich corpus of observations, the paper was marred by a complete lack of data presentation in the form of figures or tables.
Another form of learning in starfish has been called, perhaps unfortunately, ingestive conditioning, shown in Pisaster giganteus and Pisaster californianus (Landenberger, 1968), Asterias rubens (Castilla, 1972), the Crown-of-thorns starfish Acanthaster planci (Ormond et al., 1976), and Luidia clathrata (McClintock & Lawrence, 1984). Starfish have preferences when it comes to prey to consume. In all these studies, feeding the starfish with non-preferred food for a period, in the absence of other food, led to increased acceptance of and preference for the proffered food. This phenomenon might reflect some form of perceptual learning, but it is possible that starfish had also learned to better handle the proffered non-preferred food when only it was available, which would constitute a form of operant conditioning.
The Crown-of-thorns starfish feeds on corals and is often considered a threat to the preservation of these corals (see the statements of the Australian Institute of Marine Science: https://www.aims.gov.au/docs/research/biodiversity-ecology/threats/cots.html). Ormond et al. (1976) also found in the wild that this starfish preferred the more abundant types of corals, a kind of “search image” effect (Pietrewicz & Kamil, 1979), in which predators learn to better detect the most abundant type of prey. Without further study, however, such an interpretation for starfish is far from certain.
Not all learning studies on echinoderms show clear positive evidence. A study on circadian patterns in anticipating daily feeding time in Japanese sea cucumbers, Apostichopus japonica (a food source in Asia), found only slim evidence (Yamaguchi et al., 2016). The animals were fed from an automatic dispenser at either 00:00 h or 12:00 h daily. Activity was measured as the dependent variable. Only adults fed at 00:00 h showed a circadian pattern of activity with most activity around the feeding time. Juveniles’ activity level did not differ across the day no matter what the feeding time. It should be noted, however, that activity was measured across 6-h blocks, coarse blocks of time, and the sample size was small in the study.
As mentioned earlier, a phenomenon suggesting memory was discovered by Jennings (1907) in the movements of starfish. Now called directional memory, this phenomenon was investigated in the 21st century by Yoshimura et al. (2012, 2018), testing sea urchins Hemicentrotus pulcherrimus. The sea urchins were placed in the center of a square aquarium and allowed to travel until they reached an edge (Yoshimura et al., 2012). The echinoderms then inevitably moved until they ended up in a corner. After resting 5 min at the corner, they were picked up and placed in a random orientation at the center of a circular arena. Looking across the test animals, the arms that led the move were random, but in each animal, the leading arms were the ones that were touching the corner. If the sea urchin was turned at the corner in the square aquarium, and then left for 5 min, it was the arms that were last in contact with the corner that led the movement in the circular arena. The way the sea urchin was picked up and turned did not influence the results. This kind of memory is thus egocentrically based. It is memory of a particular arm, or arms of the body, as opposed to some direction in allocentric space.
Further unpublished results suggested that the tube feet of an arm needed to touch the wall to establish such a directional memory; simply having the spines of the arm touch the wall was insufficient. But the group’s follow-up study (Yoshimura et al., 2018) contradicted this. Here, memory of a body part was investigated, in which the term “directional memory” was coined (Yoshimura et al., 2018). It turned out that the tube feet did not have to touch a wall to establish this kind of memory, contradicting the group’s earlier unpublished findings mentioned in Yoshimura et al. (2012). Just moving in a particular direction, with one or more arms leading, was sufficient. Post-delay, the sea urchin was likely to lead again with the same arms. Memory is unlikely to be kept by keeping some tube feet moving, because all the tube feet retracted when the animals were picked up, possibly to avoid desiccation. The directional memory persisted with a 5-min delay period, but not with a 10-min or 30-min delay period.
Directional memory remained intact even when the top third of the sea urchin was lopped off, including the viscera, exposing the nerve ring and radial nerves. This allowed the authors to manipulate and dissect the nervous system, literally, in their investigation (Yoshimura et al., 2018). The topless sea urchin was allowed to start moving in a particular direction, and then it was picked up and radial nerves were cut midway or at their junction with the nerve ring. Because the original operation to take the top off had lesioned some of the ends of the radial nerves, the midway cut left about 1/3 of the radial nerve. Not surprisingly, the nervous system was implicated in directional memory. Cutting all the radial nerves at their junction with the nerve ring led to no more movement on the part of the sea urchins. Nevertheless, even with all 5 radial nerves reduced to one third, the sea urchins still exhibited directional memory. Leaving some nerves uncut while cutting others, however, typically distorted directional memory, in the sense that the uncut nerves typically dominated proceedings and led the way.
Reminiscent of Descartes’ (1991, p. 146) idea that a skilled lutenist might possess memory in the hands, Yoshimura et al. (2018) conjectured that directional memory in sea urchins might be, in a sense, in the tube feet. In Yoshimura et al.’s (2018) model, all the tube feet on one arm are coordinated to carry out one of four functions, a four-gear system. They could lead, which means move so as to propel the animal in the direction of the arm, they might trail, which means move so as to support another leading arm, they might attach to the substrate, or they might retract. Directional memory could come about if the tube feet of the former leading arm somehow ‘remembered’ to move first post delay, thus metaphorically dragging the other arms to support their leading movements. But the authors also admitted that the nerve-ring-and-radial-nerve complex might also support directional memory. This phenomenon of directional memory in sea urchins invites further neuroethological study.
Expanding neuroecology: comparisons across phyla
A key component of our call for an expanded view of neuroecology would be the cataloguing and comparison of cognitive abilities across a wide range of phyla. Such broad phylogenetic comparisons are vital to uncovering the ecological forces that drive the need for learning and memory as well as our understanding of how and why varied neural systems and in particular the centralized brain arose along the evolutionary timeline. Categorizing cognitive abilities that can be housed outside the brain, as well as the limitations of these embodied systems can also reveal for which abilities the presence of a brain may be critical. In the spirit of this call to action for an expanded neuroecology beyond the brain, here we compare cognitive evidence in echinoderms to their closest relative groups within Deuterostomia, namely chordates and hemichordates. Such comparisons can be informative in illuminating the evolution of learning and memory, revealing when cognitive abilities first arose.
Hemichordates are a group of about 120 wormlike marine animal species that represents a sister group to echinoderms and little is known about their cognitive abilities. By contrast, much is known of cognition in Chordata, at least the vertebrates within that phylum, and a thorough examination would be well beyond this review. (Tunicates and lancelets are spineless chordate animals.) We chose instead to focus briefly on a few of the advanced cognitive abilities present in fish, as they represent both the most common vertebrate group (~32,000 species) and the oldest lineage within vertebrates. Cognitive abilities shared between fish and other vertebrates can be considered part of a common cognitive tool box within the group and inform when these abilities arose phylogenetically, especially when such abilities are currently thought to be beyond the cognitive limitations of echinoderms.
Fish are often overlooked in discussions of animal cognition, based on misguided views of a linear evolutionary progression (Hodos & Campbell, 1969), yet they exhibit a rich variety of complex behaviors and cognitive abilities (C. Brown, 2015; C. Brown et al., 2006), including many that go well beyond the established cognitive abilities of echinoderms. Fish show ample evidence of non-associative and associative learning (both classical and operant conditioning), long-term memory retention, reversal learning, complex spatial learning, cooperative behaviors, and social learning. Of these, the documentation of spatial learning and social learning behaviors are of particular interest (C. Brown, 2015; C. Brown & Laland, 2011).
Spatial learning and memory in fish has been thoroughly explored, exceeding the directional memories documented in echinoderms and is on par with other vertebrate groups (Odling-Smee & Braithwaite, 2003). As in other vertebrates, fish can use both feature and geometry cues to orient and find objects (A.A. Brown et al., 2007; Vargas et al., 2004). Additionally, they can recognize previously visited locations via visual landmarks (Cain & Malwal, 2002; Hughes & Blight, 2000; White & Brown, 2014). Even fish species that do not use sight can encode spatial information about their environment to navigate. Blind cave fish (Anoptichthys jordani) can navigate using their lateral line organ (a sensory system that detects water movements bouncing off environmental objects) to learn and retain spatial information (de Perera, 2004; Teyke, 1989). Fish can learn multiple characteristics and relationships between cues in their spatial environments. Researchers suggest that fish can form cognitive maps of their environments (C. Brown, 2015) despite lacking a hippocampal formation, a region that in the mammalian brain supports spatial learning and memory formation. Spatial learning and memory in fish is instead supported by neural structures within the telencephalon, which is considered a hippocampal homolog (Mueller & Wullimann, 2009). Rock-pool gobies live in ephemeral pools in the intertidal zone and must quickly find their home pool before the tide makes it inaccessible. This group of gobies exhibit enhanced spatial capabilities as well as larger telencephalon regions compared to their relatives, sand-dwelling gobies that do not return to home pools with the tides (Bshary & Brown, 2014; White & Brown, 2014).
Fish have been observed to possess exceptional long-term memory capacities for spatial information. The aforementioned rock-pool gobies, likely aided by their enhanced telencephalon, learn complex spatial environments and can return to their home pool after 30-m displacements. Additionally, these home-range memories are extremely stable, with these gobies able to return to their home ranges after 40 days without exposure (Aronson, 1956; White & Brown, 2014). On the extreme end, exceptionally long-term olfactory memories are evident in Salmonids, which are born in upland rivers and as juveniles learn the specific olfactory cues of their natal river before migrating out to sea. As adults they can return to the mouth of this specific river using these long-term memories after multiple years in the open ocean (Dittman & Quinn, 1996).
Fish also show a variety of social learning and cooperative behaviors. Learning by observing or interacting with others allows for rapid information gathering about the environment, without the need to explore fully oneself. This can be especially true of learning about novel prey types. When paired with experienced individuals, naïve Atlantic salmon juveniles will learn to select novel prey items significantly more than when alone or paired with other naïve individuals (C. Brown & Laland, 2002). Fish can also learn to exhibit novel foraging behaviors after observing others. Sea Bass juveniles quickly learn operant tasks such as manipulating a lever to receive food by observing experienced demonstrators (Anthouard, 1987). The information that can be learned from others is not limited to foraging. Naïve individuals can learn a number of tasks from observing knowledgeable conspecifics (C. Brown & Laland, 2003). Juvenile fish will follow their more experienced conspecifics to learn both foraging locations as well as migratory routes (Helfman & Schultz, 1984; Laland & Williams, 1997). Inexperienced fish can also learn to recognize potential predators by observing others in their group (C. Brown & Laland, 2011). Finally, social learning plays a key role in assessing others, both for mating and as potential rivals. Multiple fish species exhibit mate-choice copying, with an individual’s propensity for selecting a mating partner based on observing another individual’s preference for that partner (Dugatkin, 1992; Schlupp & Ryan, 1997). In assessing rivals, male bystanders can employ information about others’ fighting abilities from eavesdropping on other interactions. This allows individuals to learn social hierarchies and the ranks of individual conspecifics through observational learning without risking injury (Grosnick et al.,2007).
Across a range of cognitive skills, fish have been shown to be largely on par with other vertebrate groups, which is interesting evolutionarily as fish are the common phylogenetic ancestor to the rest of the vertebrate lineage (Bshary & Brown, 2014). Hence, the similarities evident both in the neural structures of the vertebrate brain and cognitive abilities expressed widely in vertebrates (in comparison with the current understanding of learning in Echinodermata presented here) indicate that vertebrates possess a shared cognitive toolkit that may have arisen within deuterostomes in early chordates, or at least early vertebrates.
Discussion
Learning in echinoderms is largely recorded in starfish (Asteroidea), with some scattered evidence also compiled in brittle stars (Ophiuroidea), and sand dollars and sea urchins (Echinoidea). In contrast, evidence of learning in the two remaining echinoderm classes, sea lilies (Crinoidea) and sea cucumbers (Holothuroidea), is largely absent, in the latter group, only negative evidence of circadian memories presented (Yamaguchi et al., 2016) and a single paper reporting habituation published this year (Hamel et al., 2021). Given the range of lifestyles present in this phylum, a focus on the behavior of the more mobile classes might be expected, yet even in brittle stars and sea urchins there is scarce attention to learning in the literature. Starfish have received, by far, the most attention in cataloging learning, showing evidence of non-associative learning both in sensitization and habituation (Jennings, 1907). Additionally, starfish also exhibit a variety of associative learning in the form of avoidance learning, food conditioned phototaxis, and inhibitory learning. Some evidence of operant learning in starfish is present in the classical literature (Jennings, 1907; Ven, 1921). Given the lack of statistical analysis and repeatability issues, however, these capabilities remain speculative. Beyond starfish, members of the class Echinoidea also show evidence of both non-associative and associative learning, with sand dollars exhibiting sensitization (Smith & Smith, 1985), while sea urchins have been shown to exhibit directional memory (Yoshimura et al., 2012, 2018). Classical literature also contains examples of both operant learning and directional memory in brittle stars (Cowles, 1910; Preyer, 1887), but these findings are controversial and are contradicted elsewhere (Glaser, 1907; Willows & Corning, 1975), a reason we have not reviewed them. A comprehensive view of echinoderm cognition is lacking given the scarce research in multiple groups across the phylum, which makes general statements on echinoderm learning difficult. Even the scattered evidence concentrated mostly in starfish, however, hints at a potential myriad of cognitive abilities in this group despite lacking a brain. For neuroecology within this phylum, the only hypothesis that we would speculate on is that a more mobile and hunting lifestyle, such as those of many starfish, would be associated with learning abilities. But clearly, more scientifically rigorous and comprehensive studies of learning and memory in this phylum are warranted, especially those focused on the understudied classes as well as the potential capacity for operant learning. Fully cataloging the capacity for associative learning in echinoderms is critical for our understanding of evolutionary transitions in learning (Ginsburg & Jablonka, 2021), a theme that we pick up later after a call for broadening neuroecology.
Broadening neurocology beyond the brain: embodied cognition
Learning has often been described as any modification of behavior based on experience in a relevant way (Shettleworth, 2010), with qualifications to rule out the likes of fatigue, injury, or growth, yet there lurks an assumption that some underlying neural change occurs, typically in the brain, that is observable through these behavioral changes (West-Eberhard, 2003). Learning in Echinodermata shows, however, that both associative and non-associative learning can be supported without the presence of a brain, with learning and memory centers distributed throughout the animal’s body; in echinoderms evidence points to coordination centers in the radial nerve junctions or the tube feet themselves. Evidence of such distributed cognitive functions contradicts the still dominant view that cognition is all centralized in the brain. One sense of embodied cognition is any form of cognition orchestrated principally outside of a central brain (Cheng, 2018; Hochner, 2012; Keijzer, 2017; Lyon, 2019; Smith-Ferguson & Beekman, 2019). In this sense, any learning in a group such as Echinodermata that lacks a brain would constitute embodied cognition.
Even animals with brains off-load some of their cognitive load outside of the brain. Learning centers housed outside the brain have long been observed in insects. Horridge (1962) reported associative learning to avoid electric shocks in headless cockroaches. Here, the embodied learning sites were within the prothoratic ganglia of the animal’s ventral nerve cord (Eisenstein & Cohen, 1965; Horridge, 1962). Cephalopods (phylum Mollusca) have centralized brains and exhibit a wide range of learning and memory abilities, yet these animals also relegate some coordination of behaviors to the periphery. In cuttlefish, neural control of their camouflage is largely distributed into organs near the skin (Chiao et al., 2015). In the octopus, each arm has the ability to both bend at any location and grasp food using suction cups at any location along its ventral surface, giving each arm almost infinite degrees of freedom of movement. Too many degrees of freedom spells difficulties for central control. The act of moving food from the arm to the animal’s mouth is controlled via the arms themselves. The animal’s peripheral nervous system transforms the arm’s muscles to become a temporary quasi-articulated elbow joint with a hinge at the midpoint between the food and the base of the arm coming out of the body, thus allowing the food piece to be brought to the mouth by bending the elbow (Flash & Hochner, 2005; Hochner, 2012).
In the spirit of the broader neuroecology we are promoting, we now consider briefly what forms of learning are found in other organisms lacking a brain and even lacking nervous systems altogether. An abundance of literature shows that another group of organisms with nerve nets show non-associative and associative learning: cnidarians (corals, sea anemones, hydras, box jellies, and true jellyfish; Cheng, 2021). This phylum evolved a diffuse interconnection of neurons without cephalization or a central brain, independently of echinoderms (L.Z. Holland et al., 2013; N. Holland, 2003). Hydras, jellyfish, and sea anemones all exhibit non-associative learning while possessing only minimal centralization and coordination within their nerve rings (Cheng, 2021; Satterlie, 2011). Evidence of associative learning, however, is much clearer in echinoderms than in cnidarians (Cheng, 2021). In Cnidaria, only a single well-structured study on sea anemones provides solid evidence for classical conditioning (Haralson et al., 1975), which leaves doubts about the robustness of the evidence (Bronfman et al., 2016; Cheng, 2021; Ginsburg & Jablonka, 2010).
Spreading the neuroecological perspective wider, even non-neural organisms, including single-celled organisms, display learning, at least non-associative learning, although evidence for associative learning in these phyla remains dubious or at least contentious (Baluška & Levin, 2016; Dussutour, 2021; Gagliano et al., 2014). The touch-me-not Mimosa pudica habituates in its reactions to repeated provoking stimuli (Box 1). The single-celled slime mold Physarum polycephalum also displays habituation to initially aversive stimuli (Box 1).
————————————————————————
Box 1 Non-associative learning in non-neural organisms
Evidence can be found for non-associative learning in single-celled organisms such as slime molds and in plants, but no convincing evidence for associative learning is currently at hand (single-celled organisms: Dussutour, 2021; plants: Castiello, 2021). While Castiello (2021) reviewed one study showing associative learning in pea plants (Gagliano et al., 2016), a replication with a larger sample size failed to produce positive results (Markel, 2020). Dussutour’s (2021) review doubted all the evidence so far for associative learning in single-celled organisms, and such doubts are even expressed by those sympathetic to the idea of associative learning in such organisms (Gershman et al., 2021). We will not review this contentious topic. Instead, we describe two studies showing habituation in plants (Gagliano et al., 2014) and slime molds (Boisseau et al., 2016).
The mimosa Mimosa pudica usually folds its leaves up when disturbed. In the study on habituation, M. pudica plants were repeatedly dropped in a controlled fashion and their leaf folding was measured (Gagliano et al., 2014). The plants slid down a vertical rail from a designated height to land on a foam-cushioned surface. Repeated sessions of 60 drops were imposed on the plants. The plants reduced their leaf folding with these multiple drops, interestingly, to different extents in different light environments. They habituated more (less leaf folding) in a low-light environment than in a high-light environment. With multiple sessions of 60 drops, long-term habituation up to 28 days was found. The habituation showed stimulus specificity in that when a different kind of disturbance, shaking, was instituted, leaf folding was once again found. Stimulus specificity or dishabituation is important for ruling out fatigue as an explanation for diminished responding. A weakness in the study was a lack of counterbalancing of the habituating stimulus: Habituation with repeated shaking was not examined.
The large, amoeba-like slime mold Physarum polycephalum was used to study habituation (Boisseau et al., 2016). These single-celled organisms can span meters. The slime molds had to cross a bridge laced with the initially aversive chemicals caffeine or quinine to reach a food source. A number of dependent measures were taken as indices of aversion, including the time to first contact the bridge, the time to cross the bridge, and the shape of the pseudopod contacting the bridge, with a slim finger indicating aversion and a rounded, “full-frontal” assault indicating a lack of aversion. Generally, measures of aversion diminished over days of exposure, but recovered to different extents after a two-day break in which only agar covered the bridge. Stimulus specificity was also found by switching between caffeine and quinine. This study fully counterbalanced caffeine and quinine as habituating stimuli and included control slime molds exposed to agar only in training.
——————End of Box 1——————
Evolutionary perspectives on learning
Ginsburg and Jablonka (2021) recognize five transitions in the evolution of learning, with the first two transitions occurring before the need for higher-level brain organization. The first is the transition from learning in non-neural systems to neural systems, with learning in non-neural organisms linked to epigenetic molecular mechanisms (Gershman et al., 2021; Ginsburg & Jablonka, 2009, 2021; Langille & Gallistel, 2020). One example would be memory encoded in markings on DNA in the form of chemical components such as methyl groups added to a string of DNA, the process of methylation. Ginsburg and Jablonka (2009) conjectured that such mechanisms can provide a memorial code even for single cells; they made what they called toy models that support habituation and sensitization, among others (Ginsburg & Jablonka, 2009). The second evolutionary transition is the ability of limited associative learning, linked to a centralized nervous system. Here, Ginsburg and Jablonka (2021) state that both bilateral symmetry and a centralized brain are key to the evolution of associative learning and dispute a scattering of conditioning evidence in organisms outside of the bilaterians, including in Cnidaria (Cheng, 2021; Haralson et al., 1975). Within the bilaterian lineage, however, the associative learning abilities evidenced in echinoderms would suggest that the lower levels of neural centralization within echinoderms may be sufficient.
Such evidence of non-associative and associative learning in echinoderms with only limited amounts of neural centralization should also make clear the need to uncover the diverse mechanisms by which these cognitive abilities are supported. The neural circuits of the hippocampal region of the brain in mammals (Rowland et al., 2016), the telencephalon in fish (Mueller & Wullimann, 2009), or the central complex in invertebrate insects (Heinze et al., 2018) cannot be proposed for learning in brainless organisms such as echinoderms. In the absence of these neural mechanisms, what supports learning in brainless organisms? Sites of embodied cognition in echinoderms, specifically the radial nerve junctions, deserve further neurobiological research as structures that could support learning and memory. An alternative proposed mechanism is that memory may have a chemical basis in the DNA and RNA of organisms, such as in the epigenetic mechanisms envisaged by Ginsburg and Jablonka (2009, 2021, see also Gershman et al., 2021; Langille & Gallistel, 2020). The basic idea of all such proposals is that memorial records are stored in a readable form on molecules, as opposed to being instantiated in the activities of neural (or other) circuits (Gershman et al., 2021; Langille & Gallistel, 2020). Metaphorically, this form of memory is akin to having a physical signpost telling hikers where to head on a trail, compared with having a team of workers giving out instructions; the former is much, much cheaper. Such potential for learning and memory to be supported by molecular architectures would suggest that cognitive abilities could be possessed by a wide range of organisms, including learning in single-celled non-neural systems.
Comparing echinoderm learning to learning and cognition in vertebrates (fish) on the one hand, and to learning in Cnidaria and non-neural organisms on the other hand, has stretched the boundaries and perhaps the meaning of neuroecology. Neuroecology has been, thus far, focused on adaptive variations in cognition and the brain (Sherry, 2006). We are by no means casting doubts or criticisms on this enterprise. On the contrary, we are embracing the idea and call for pushing its boundaries beyond the brain, even to non-neural organisms. Studying brainless and non-neural organisms and what they are capable of is crucial for understanding what nervous systems in general, and what brains in particular, contribute to animals and also how and why nervous systems arose in evolution in the first place, all issues for an expanded neuroecology. As might be expected from a Darwinian perspective of evolution, neural elements did not arise de novo but are adaptations of elements found in non-neural organisms (see, for example, Arendt, 2021; Moroz et al., 2021; and other articles in the special issue containing these references). Thus, neuroecology should be pushed beyond learning and memory housed in the brain and include both animals with learning centers that are embodied as well as non-neural organisms.
Conclusions
A thorough cataloging of the cognitive capabilities as well as limitations across animal phyla is critical to understanding how abilities such as learning and memory are distributed across a range of neural architectures, as well as unraveling how cognition evolved in animal lineages (Perry et al., 2013). Non-associative learning shows ample evidence of not requiring the presence of a centralized brain, as multiple phyla show this type of learning, including habituation in single-celled slime molds (Boisseau et al., 2016) and in plants (Gagliano et al., 2014), as well as both habituation and sensitization in cnidarians (Cheng, 2021) and echinoderms (current paper). While clear evidence of associative learning is largely absent in these other groups, evidence in echinoderms indicates that within the Deuterostome phyla, the presence of a brain is also unnecessary for associative learning and memories. Echinoderms show evidence of both in the form of food conditioning, inhibition, and directional memories. Cognitive abilities such as reinforcement learning and avoidance behavior are hinted at in the classical literature, although the evidence for these abilities is marred by the lack of rigorous data collection and inferential statistics, while other examples are contradicted by later work, making any firm conclusions impossible. Clearly, further study in echinoderm learning is warranted as part of a broadening of the neuroecology that Sherry (2006) championed.
Data availability
None of the data or materials for the experiments reviewed here are available, and none of the experiments were preregistered. No new original data are reported.
References
Andrew, N., & Underwood, A. (1993). Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Marine Ecology Progress Series, 99, 89–98.
Anthouard, M. (1987). A study of social transmission in juvenile Dicentrarchus labrax (Pisces, Serranidae), in an operant conditioning situation. Behaviour, 103(4), 266–275.
Arendt, D. (2021). Elementary nervous systems. Philosophical Transactions of the Royal Society B, 376(1821), 20200347.
Aronson, L. R. (1956). Further studies on orientation and jumping behavior in the goby fish, Bathygobius soporator. Anatomical Records, 125, 606.
Arshavskii, Y. I., Kashin, S. M., Litvinova, N. M., Orlovskii, G. N., & Fel'dman, A. G. (1976). Coordination of arm movement during locomotion in ophiurans. Neurophysiology, 8, 404–410.
Astley, H. C. (2012). Getting around when you're round: quantitative analysis of the locomotion of the blunt-spined brittle star, Ophiocoma echinata. Journal of Experimental Biology, 215, 1923–1929.
Baluška, F., & Levin, M. (2016). On having no head: Cognition throughout biological systems. [Review]. Frontiers in Psychology, 7, 902. https://doi.org/10.3389/fpsyg.2016.00902
Beddingfield, S. D., & McClintock, J. B. (1993). Feeding behavior of the sea star Astropecten articulatus (Echinodermata: Asteroidea): an evaluation of energy-efficient foraging in a soft-bottom predator. Marine Biology, 115(4), 669–676.
Birenheide, R., Motokawa, T. (1996). Contractile connective tissue in crinoids. The Biological Bulletin, 191(1), 1–4.
Boisseau, R. P., Vogel, D., & Dussutour, A. (2016). Habituation in non-neural organisms: evidence from slime moulds. Proceedings of the Royal Society B: Biological Sciences, 283(1829), 20160446.
Bronfman, Z. Z., Ginsburg, S., & Jablonka, E. (2016). The transition to minimal consciousness through the evolution of associative learning. [Hypothesis and Theory]. Frontiers in Psychology, 7, 1954. https://doi.org/10.3389/fpsyg.2016.01954
Brown, C. (2015). Fish intelligence, sentience and ethics. Animal cognition, 18(1), 1–17.
Brown, C., & Laland, K. N. (2002). Social enhancement and social inhibition of foraging behaviour in hatchery-reared Atlantic salmon. Journal of Fish Biology, 61(4), 987–998.
Brown, C., & Laland, K. N. (2003). Social learning in fishes: a review. Fish and fisheries, 4(3), 280–288.
Brown, C., & Laland, K. (2011). Social learning in fishes. In In C. Brown, K. Laland, & J. Krause (Eds.), Fish cognition and behavior (pp. 240–257). John Wiley & Sons.
Brown, C., Laland, K., & Krause, J. (2006). Fish cognition and behavior (Vol. 21). John Wiley & Sons.
Brown, A. A., Spetch, M. L., & Hurd, P. L. (2007). Growing in circles: Rearing environment alters spatial navigation in fish. Psychological Science, 18(7), 569–573.
Bshary, R., & Brown, C. (2014). Fish cognition. Current Biology, 24(19), R947–R950.
Cain, P., & Malwal, S. (2002). Landmark use and development of navigation behaviour in the weakly electric fish Gnathonemus petersii (Mormyridae; Teleostei). Journal of Experimental Biology, 205(24), 3915–3923.
Cameron, C. B., Garey, J. R., & Swalla, B. J. (2000). Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proceedings of the National Academy of Sciences of the United States of America, 97, 4469–4474.
Castiello, U. (2021). (Re)claiming plants in comparative psychology. Journal of Comparative Psychology, 135, 127–141.
Castilla, J. C. (1972). Responses of Asterias rubens to bivalve prey in a Y-maze. Marine Biology, 12(3), 222–228.
Cheng, K. (2016). How animals think and feel. ABC-CLIO.
Cheng, K. (2018). Cognition beyond representation: Varieties of situated cognition in animals. Comparative Cognition & Behavior Reviews, 13, 1–20.
Cheng, K. (2021). Learning in Cnidaria: A systematic review. Learning & Behavior, 1–15.
Chiao, C.C., Chubb, C., & Hanlon, R. T. (2015). A review of visual perception mechanisms that regulate rapid adaptive camouflage in cuttlefish. Journal of Comparative Physiology A, 201, 933–945.
Clark, E. G., Kanauchi, D., Kano, T., Aonuma, H., Briggs, D. E. G., & Ishiguro, A. (2019). The function of the ophiuroid nerve ring: how a decentralized nervous system controls coordinated locomotion. The Journal of Experimental Biology, 222(2), jeb192104.
Cobb, J. L. S. (1987). Neurobiology of the Echinodermata. In M. A. Ali (Ed.), Nervous systems in invertebrates (pp. 483–525). Plenum Press.
Cobb, J. L. S. (1995). The nervous systems of Echinodermata: Recent results and new approaches. In: Experientia Supplementum (pp. 407–424). Birkhäuser Basel.
Cowles, R. P. (1910). Stimuli produced by light and by contact with solid walls as factors in the behavior of ophiuroids. Journal of Experimental Zoology, 9(2), 387–416.
De Perera, T. B. (2004). Fish can encode order in their spatial map. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271(1553), 2131–2134.
Descartes, R. (1991). The philosophical writings of Descartes (J. Cottingham, R. Stoothoff, D. Murdoch & A. Kenny, Trans. Vol. III The correspondence). Cambridge University Press.
Diebschlag, E. (1938). Ganzheitliches Verhalten und Lernen bei Echinodermen. Zeitschrift für vergleichende Physiologie, 25(4), 612–654.
Dittman, A., & Quinn, T. (1996). Homing in Pacific salmon: mechanisms and ecological basis. Journal of Experimental Biology, 199(1), 83–91.
Domenici, P., González-Calderón, D., & Ferrari, R. S. (2003). Locomotor performance in the sea urchin Paracentrotus lividus. Journal of the Marine Biological Association of the United Kingdom, 83(2), 285–292.
Dugatkin, L. A. (1992). Sexual selection and imitation: females copy the mate choice of others. The American Naturalist, 139(6), 1384–1389.
Dussutour, A. (2021). Learning in single cell organisms. Biochemical and Biophysical Research Communications, 564, 92–102.
Eisenstein, E. M., & Cohen, M. J. (1965). Learning in an isolated prothoracic insect ganglion. Animal Behaviour, 13(1), 104–108.
Emson, R. H., Mladenov, P. V., & Barrow, K. (1991). The feeding mechanism of the basket star Gorgonocephalus arcticus. Canadian Journal of Zoology, 69(2), 449–455.
Flammang, P. (1996). Adhesion in echinoderms. Echinoderm Studies, 5, 1–60.
Flash, T., & Hochner, B. (2005). Motor primitives in vertebrates and invertebrates. Current Opinion in Neurobiology, 15, 660–666.
Gagliano, M., Renton, M., Depczynski, M., & Mancuso, S. (2014). Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia, 175(1), 63–72.
Gagliano, M., Vyazovskiy, V. V., Borbély, A. A., Grimonprez, M., & Depczynski, M. (2016). Learning by association in plants. Scientific reports, 6(1), 1–9.
Gershman, S. J., Balbi, P. E. M., Gallistel, C. R., & Gunawardena, J. (2021). Reconsidering the evidence for learning in single cells. Elife, 10, e61907.
Ginsburg, S., & Jablonka, E. (2009). Epigenetic learning in non-neural organisms. Journal of Biosciences, 34(4), 633–646.
Ginsburg S., & Jablonka E. (2010). The evolution of associative learning: a factor in the Cambrian explosion. Journal of Theoretical Biology, 266, 11–20.
Ginsburg, S., & Jablonka, E. (2021). Evolutionary transitions in learning and cognition. Philosophical Transactions of the Royal Society B, 376(1821), 20190766.
Glaser, O. C. (1907). Movement and problem solving in Ophiura brevispina. Journal of Experimental Zoology, 4(2), 203–220.
Grimmer, J. C., Holland, N. D. (1987). The role of ligaments in arm extension in feather stars (Echinodermata: Crinoidea). Acta Zoologica, 68(2), 79–82.
Grosenick, L., Clement, T. S., & Fernald, R. D. (2007). Fish can infer social rank by observation alone. Nature, 445(7126), 429–432.
Güler, M., & Lök, A. (2015). Foraging behaviors of sea stars, Marthasterias glacialis and Astropecten aranciacus (Asteroidea) and predator–prey interactions with warty venus clam, Venus verrucosa (Bivalvia). Journal of Experimental Marine Biology and Ecology, 465, 99–106.
Hamel, J.-F., Jobson, S., Caulier, G., & Mercier, A. (2021). Evidence of anticipatory immune and hormonal responses to predation risk in an echinoderm. Scientific Reports, 11(1), 10691.
Hampton, R. R., Sherry, D. F., Shettleworth, S. J., Khurgel, M., & Ivy, G. (1995). Hippocampal volume and food-storing behavior are related in parids. Brain, Behavior and Evolution, 45(1), 54–61.
Haralson, J. V., Groff, C. I., & Haralson, S. J. (1975). Classical conditioning in the sea anemone, Cribrina xanthogrammica. Physiology and Behavior, 15(4), 455–460. https://doi.org/10.1016/0031-9384(75)90259-0
Heinze, S., Narendra, A., & Cheung, A. (2018). Principles of insect path integration. Current Biology, 28(17), R1043–R1058.
Helfman, G. S., & Schultz, E. T. (1984). Social transmission of behavioural traditions in a coral reef fish. Animal Behaviour, 32(2), 379–384.
Hochner, B. (2012). An embodied view of octopus neurobiology. Current Biology, 22, R887–R892.
Hodos, W., & Campbell, C. B. G. (1969). Scala naturae: Why there is no theory in comparative psychology. Psychological Review, 76(4), 337–350.
Hoekstra, L. A., Moroz, L. L., & Heyland, A. (2012). Novel insights into the echinoderm nervous system from histaminergic and FMRFaminergic-like cells in the sea cucumber Leptosynapta clarki. PLoS ONE, 7(9), e44220.
Holland, N. D. (2003). Early central nervous system evolution: an era of skin brains? Nature Reviews Neuroscience, 4(8), 617–627.
Holland, P. (2011). The animal kingdom: A very short introduction. Oxford University Press.
Holland, L. Z., Carvalho, J. E., Escriva, H., Laudet, V., Schubert, M., Shimeld, S. M., & Yu, J.-K. (2013). Evolution of bilaterian central nervous systems: a single origin? EvoDevo, 4(1), 27.
Horridge, G. A. (1962). Learning of leg position by the ventral nerve cord in headless insects. Proceedings of the Royal Society of London. Series B. Biological Sciences, 157(966), 33–52.
Hughes, R. N., & Blight, C. M. (2000). Two intertidal fish species use visual association learning to track the status of food patches in a radial maze. Animal behaviour, 59(3), 613–621.
Hyman, L. H. (1955). The Invertebrates: Echinodermata. The Coelomate Bilateria Vol. 4. McGraw-Hill Book Co., Inc.
Janevski, G. A., & Baumiller, T. K. (2010). Could a stalked crinoid swim? A biomechanical model and characteristics of swimming crinoids. PALAIOS, 25(9), 588–596.
Jennings, H. S. (1907). Behavior of the starfish Asterias forreri de Loriol. University of California Publications in Zoology, 4, 53–185.
Ji, C., Wu, L., Zhao, W., Wang, S., & Lv, J. (2012). Echinoderms have bilateral tendencies. PLoS ONE, 7(1), e28978.
Keijzer, F. A. (2017). Evolutionary convergence and biologically embodied cognition. Interface Focus, 7(3), 20160123.
Kerkut, G. A. (1954). The mechanisms of coordination of the starfish tube feet. Behaviour, 6(1), 206–232.
Kerkut, G. E. (1955). The retraction and protraction of the tube feet of the starfish (Asterias rubens). Behaviour, 8, 112–129.
Krebs, J. R., Sherry, D. F., Healy, S. D., Perry, V. H., and Vaccarino, A. L.. (1989). Hippocampal specializaton of food-storing birds. Proceedings of the National Academy of Sciences of the USA, 86, 1388–1392.
Laland, K. N., & Williams, K. (1997). Shoaling generates social learning of foraging information in guppies. Animal Behaviour, 53(6), 1161–1169.
Landenberger, D. E. (1966). Learning in the Pacific starfish Pisaster glganteus. Animal Behaviour, 14(4), 414–418.
Landenberger, D. E. (1968). Studies on selective feeding in the Pacific starfish Pisaster in southern California. Ecology, 49(6), 1062–1075.
Langille, J. J., & Gallistel, C. R. (2020). Locating the engram: Should we look for plastic synapses or information-storing molecules? Neurobiology of Learning and Memory, 169, 107164.
Lawrence, J. M., & Sammarco, P. W. (1982). Effects of feeding on the environment: Echinoidea. In M. Jangoux & J. M. Lawrence (Eds.), Echinoderm nutrition (499–519). AA Balkema.
Linchangco, G. V., Jr., Foltz, D. W., Reid, R., Williams, J., Nodzak, C., Kerr, A. M., ... Janies, D. A. (2017). The phylogeny of extant starfish (Asteroidea: Echinodermata) including Xyloplax, based on comparative transcriptomics. Molecular Phylogenetics and Evolution, 115, 161–170. https://doi.org/10.1016/j.ympev.2017.07.022
Lyon, P. (2019). Of what is “minimal cognition” the half-baked version? Adaptive Behavior, 28(6), 407–424.
Markel, K. (2020). Lack of evidence for associative learning in pea plants. Elife, 9, e57614.
Mashanov, V.S., Zueva, O.R., Heinzeller, T., & Dolmatov, I.Y. (2006). Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology, 125, 27–38.
Mashanov, V. S., Zueva, O. R., & Heinzeller, T. (2008). Regeneration of the radial nerve cord in a holothurian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue and Cell, 40(5), 351–372.
Mashanov, V. S., Zueva, O. R., Heinzeller, T., Aschauer, B., Naumann, W. W., Grondona, J. M., Cifuentes, M., & Garcia-Arraras, J. E. (2009). The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Frontiers in Zoology, 6(1), 11.
Mashanov, V., Zueva, O., Rubilar, T., Epherra, L., & García-Arrarás, J. E. (2016). Echinodermata. In A. Schmidt-Rhaesa, S. Harzch, & G. Purschke (Eds.) Structure and evolution of invertebrate nervous systems (pp. 665–688). Oxford University Press.
Matsuzaka, Y., Sato, E., Kano, T., Aonuma, H., & Ishiguro, A. (2017). Non-centralized and functionally localized nervous system of ophiuroids: evidence from topical anesthetic experiments. Biology Open, 6(4) 425–438. https://doi.org/10.1242/bio.019836
McClintock, J. B., & Lawrence, J. M. (1981). An optimization study on the feeding behavior of Luidia clathrata (Say) (Echinodermata: Asteroidea). Marine Behaviour and Physiology, 7, 263–275.
McClintock, J. B., & Lawrence, J. M. (1982). Photoresponse and associative learning in Ludia clathrata Say (Echinodermata, Asteroidea). Marine Behaviour and Physiology, 9(1), 13–21. https://doi.org/10.1080/10236248209378580
McClintock, J. B., & Lawrence, J. M. (1984). Ingestive conditioning in Luidia clathrata (Say) (Echinodermata: Asteroidea): effect of nutritional condition on selectivity, teloreception, and rates of ingestion. Marine & Freshwater Behaviour & Physiology, 10(3), 167–181.
McClintock, J. B., & Lawrence, J. M. (1985). Characteristics of foraging in the soft-bottom benthic starfish Luidia clathrata (echinodermata: Asteroidea): prey selectivity, switching behavior, functional responses and movement patterns. Oecologia 66, 291–298.
McCurley, S. R., & Kier, W. M. (1995). The functional morphology of starfish tube feet: The role of a crossed-fiber helical array in movement. Biology Bulletin, 188, 197–209.
Mercier, A., & Hamel, J.-F. (2008). Nature and role of newly described symbiotic associations between a sea anemone and gastropods at bathyal depths in the NW Atlantic. Journal of Experimental Marine Biology and Ecology, 358(1), 57–69.
Miller, J. E., & Pawson, D. L. (1990). Swimming sea cucumbers (Echinodermata: Holothuroidea): a survey, with analysis of swimming behavior in four bathyal species. Smithsonian Contributions to Marine Science, 35, 1–18.
Moroz, L. L. (2015). Convergent evolution of neural systems in ctenophores. Journal of Experimental Biology, 218, 598–611.
Moroz, L. L., & Kohn, A. B. (2016). Independent origins of neurons and synapses: insights from ctenophores. Philosophical Transactions of the Royal Society B, 371, 20150041.
Moroz, L. L., Romanova, D. Y., & Kohn, A. B. (2021). Neural versus alternative integrative systems: molecular insights into origins of neurotransmitters. Philosophical Transactions of the Royal Society B, 376(1821), 20190762.
Mueller, T. & Wullimann, M.F. (2009) An evolutionary interpretation of teleostean forebrain anatomy. Brain, Behavior and Evolution, 74, 30–42.
Odling-Smee, L., & Braithwaite, V. A. (2003). The role of learning in fish orientation. Fish and Fisheries, 4(3), 235–246.
Olson, D. J., Kamil, A. C., Balda, R. P., & Nims, P. J. (1995). Performance of four seed caching corvid species in operant tests of nonspatial and spatial memory. Journal of Comparative Psychology, 109(2), 173–181.
Ord, T. J., Klomp, D. A., Garcia-Porta, J., & Hagman, M. (2015). Repeated evolution of exaggerated dewlaps and other throat morphology in lizards. Journal of Evolutionary Biology, 28(11), 1948–1964.
Ormond, R. F., Hanscomb, N. J., & Beach, D. H. (1976). Food selection and learning in the crown-of-thorns starfish, Acanthaster planci (L.). Marine and Freshwater Behaviour and Physiology, 4(2), 93–105.
Pantin, C. F. A. (1935). The nerve net of the Actinozoa: I. Facilitation. Journal of Experimental Biology, 12, 119–138.
Pawson, D. L. (2007). Phylum Echinodermata. Zootaxa, 1668, 749–764.
Pentreath, V. W., & Cobb, J. L. S. (1972). Neurobiology of Echinodermata. Biological Reviews, 47(3), 363–392. https://doi.org/10.1111/j.1469-185x.1972.tb00977.x
Perry, C. J., Barron, A. B., & Cheng, K. (2013). Invertebrate learning and cognition: relating phenomena to neural substrate. Wiley Interdisciplinary Reviews: Cognitive Science. https://doi.org/10.1002/wcs.1248.
Pietrewicz, A. T., & Kamil, A. C. (1979). Search image formation in the blue jay (Cyanocitta cristata). Science, 204(4399), 1332–1333.
Pinto, L., & Gotz, M. (2007). Radial glial cell heterogeneity—The source of diverse progeny in the CNS. Progress in Neurobiology, 83(1), 2–23.
Piscopo, S., Stefano, R. D., Thorndyke, M. C., & Brown, E. R. (2005). Alteration and recovery of appetitive behaviour following nerve section in the starfish Asterias rubens. Behavioural Brain Research, 164(1), 36–41.
Preyer, W. T. (1887). Die Bewegungen der Seesterne. Friedländer.
Rowland, D. C., Roudi, Y., Moser, M.-B., & Moser, E. I. (2016). Ten years of grid cells. Annual Review of Neuroscience, 39, 19–40.
Roy, A., Metaxas, A., & Ross, T. (2012). Swimming patterns of larval Strongylocentrotus droebachiensis in turbulence in the laboratory. Marine Ecology Progress Series, 453, 117–127.
Rutman, J., & Fishelson, L. (1969). Food composition and feeding behavior of shallow-water crinoids at Eilat (Red Sea). Marine Biology, 3, 46–57.
Satterlie, R. A. (2011). Do jellyfish have central nervous systems? Journal of Experimental Biology, 214(8), 1215–1223. https://doi.org/10.1242/jeb.043687
Schlupp, I., & Ryan, M. J. (1997). Male sailfin mollies (Poecilia latipinna) copy the mate choice of other males. Behavioral Ecology, 8(1), 104–107.
Sherry, D. F. (2006). Neuroecology. Annual Review of Psychology, 57, 167–197.
Sherry, D. F., Vaccarino, A. L., Buckenham, K., & Herz, R. S. (1989). The hippocampal complex of food-storing birds. Brain, Behavior and Evolution, 34, 308–317.
Shettleworth, S. J. (2010). Cognition, evolution, and behavior, 2nd edn. Oxford University Press.
Shulgina, G. I. (2006). Learning of inhibition of behavior in the sea star Asterias rubens. Journal of Evolutionary Biochemistry and Physiology, 42(2), 161–165. https://doi.org/10.1134/s0022093006020074
Smith, J. E. (1937). The structure and function of the tube feet in certain echinoderms. Journal of the Marine Biological Association of the United Kingdom, 22(1), 345–357.
Smith, S. L., & Smith, A. C. (1985). Sensitization and histamine release by cells of the sand dollar, Mellita quinquiesperforata. Developmental & Comparative Immunology, 9(4), 597–603.
Smith-Ferguson, J., & Beekman, M. (2019). Who needs a brain? Slime moulds, behavioural ecology and minimal cognition. Adaptive Behavior, https://doi.org/10.1177/1059712319826537.
Sokolov, V. A., (1961). Tactile conditioning in the starfish Asterias rubens, Murmanskii morskoi Biologicheskii Institut. Trudy, 3, 49–54.
Strathmann, R. R. (1971). The feeding behaviour of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of suspension feeding. Journal of Experimental Marine Biology and Ecology, 6, 109–160.
Teyke, T. (1989). Learning and remembering the environment in the blind cave fish Anoptichthys jordani. Journal of Comparative Physiology A, 164(5), 655–662.
The New Student's Reference Work (1914). Beach, B.C. & McMurry, F.M (Eds.) F.E. Compton & Company.
Valentinčič, T. (1978). Learning in the starfish Marthasterias glacialis. In D. S. McLusky & A. J. Berry (Eds.), Physiology and behaviour of marine organisms: Proceedings of the 12th European Symposium on Marine Biology, Stirling, Scotland, September 1977 (pp. 303–309). Pergamon Press.
Valentinčič, T. (1980). Associative learning in the starfish Marthasterias glacialis, a simple model for the study of learning. In M. Jangoux (Ed.), Echinoderms — present and past. Proceedings of the European Colloquium on Echinoderms, Brussels September 1979 (pp. 337–341). A. A. Balkema.
Valentinčič, T. (1985). Behavioral study of chemoreception in the sea star Marthasterias glacialis: Structure-activity relationships of lactic acid, amino acids, and acetylcholine. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology, 157(4), 537–545. https://doi.org/10.1007/bf00615155
Vargas, J. P., López, J. C., Salas, C., & Thinus-Blanc, C. (2004). Encoding of geometric and featural spatial information by goldfish (Carassius auratus). Journal of Comparative Psychology, 118(2), 206–216.
Ven, C. D. (1921). Sur la formation d'habitudes chez les asteries. Archives Neerlandaises de Physiologie., 6, 163–178.
West-Eberhard, M. J. (2003). Developmental plasticity and evolution. Oxford University Press.
White, G. E., & Brown, C. (2014). A comparison of spatial learning and memory capabilities in intertidal gobies. Behavioral Ecology and Sociobiology, 68(9), 1393–1401.
Willows, A. O. D., & Corning, W. C. (1975). The Echinorderms. In W. C. Corning, J. A Dyal, & A. O. D. Willows (Eds.), Invertebrate Learning (pp. 103–135). Springer US.
Yamaguchi, M., Masuda, R., & Yamashita, Y. (2016). Diel activity of the sea cucumber Apostichopus japonicus is affected by the time of feeding and the presence of predators but not by time–place learning. Fisheries Science, 82(1), 29–34.
Yoshimura, K., Iketani, T., & Motokawa, T. (2012). Do regular sea urchins show preference in which part of the body they orient forward in their walk? Marine biology, 159(5), 959–965.
Yoshimura, K., Tsurimaki, H., & Motokawa, T. (2018). Memory of direction of locomotion in sea urchins: effects of nerves on direction and activity of tube feet. Marine Biology, 165(5). https://doi.org/10.1007/s00227-018-3342-y
Acknowledgements
We thank David Sherry and Marcia Spetch for comments on earlier versions of this paper. We would like to thank John Elias of Macquarie University Library for his help in literature search.
Funding
This work has been partially supported by AUSMURIB000001 associated with ONR MURI grant N00014-19-1-2571 (KC) and by a Natural Sciences and Engineering Research Council of Canada Discovery grant (#2020-03933).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal ethics
As the article contains no reports of original research, regulations regarding animal ethics do not apply.
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Freas, C.A., Cheng, K. Neuroecology beyond the brain: learning in Echinodermata. Learn Behav 50, 20–36 (2022). https://doi.org/10.3758/s13420-021-00492-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-021-00492-3