Abstract
Joint presentations of a conditioned stimulus (CS) and an unconditioned stimulus (US) strengthen the contingency between them, whereas presentations of one stimulus without the other degrade this contingency. For example, the CS can be presented without the US either before conditioning (CS–no US and then CS–US; latent inhibition) or after conditioning (CS–US and then CS–no US; extinction). In vertebrate subjects and several invertebrate species, a time lapse usually results in a return of the conditioned response, or spontaneous recovery. However, in land mollusks, spontaneous recovery from extinction has only recently been reported, and response recovery after latent inhibition has not been reported. In two experiments, using conditioned aversion to a food odor as a measure of learning and memory retention, we observed contingency degradation via latent inhibition (Experiment 1) and extinction (Experiment 2) in the common garden slug, Lehmannia valentiana. In both situations, the contingency degradation procedure successfully attenuated conditioned responding, and delaying testing by several days resulted in recovery of the conditioned response. This suggests that the CS–US association survived the degradation manipulation and was retained over an interval of several days.
Similar content being viewed by others
Pavlov (1927) reported that pairings of an initially neutral stimulus with an unconditioned stimulus (US) resulted in the initially neutral stimulus coming to elicit a response consistent with the US, thereby becoming a conditioned stimulus (CS). Since the time of Pavlov, this classical conditioning procedure has been viewed as a basic process of adaptation to the environment, with many survival functions (see, e.g., Hollis, 1997; Rescorla, 1988).
The development of a conditioned response is at least partially dependent on the contingency between the CS and US (see, e.g., Rescorla, 1967). Contingencies increase with the number of instances in which the CS and US occur together, and decrease with the number of instances in which the CS occurs without the US. For example, in the so-called extinction paradigm, subsequent to CS–US pairings, the CS is presented but the US is omitted (i.e., CS–US and then CS–no US; cf. Pavlov, 1927) and conditioned responding decreases in proportion to contingency degradation. Time lapses after extinction can lead to spontaneous recovery of the response (Pavlov, 1927; Rescorla, 2004), suggesting that the CS–US association survives the contingency degradation procedure (Rescorla, 2001). Furthermore, it suggests that a CS can support multiple associations, depending on the circumstances in which those associations were acquired (Bouton, 1993). Contingency-degrading exposure to the CS can also occur before CS–US pairings, and the initial CS–no US presentations decrease the rate of acquisition or expression of the subsequently trained CS–US association. This latent inhibition effect (Lubow & Moore, 1959) has been described as a basic example of the normal functioning of attention in which the preexposed CS comes to be ignored because it does not predict any relevant information. However, latent inhibition is also subject to spontaneous recovery (see, e.g., Kraemer & Spear, 1992), suggesting that the acquisition of the CS–US association is not prevented, nor is the CS ignored, during CS–US pairings. Rather, performance based on the CS–US association appears to be impaired by the previous exposure to the CS without the US (see, e.g., Escobar, Arcediano, & Miller, 2002). Thus, latent inhibition and extinction appear to represent parallel behavioral phenomena (see, e.g., Pineño & Miller, 2005), in which expression of a CS–US association is impaired by pre- and postexposure to the CS, respectively.
CS pre- and postexposure reflect not only sensitivity to the CS–US contingency, but also reflect cognitive flexibility and the acquisition of multiple associations that are stored in memory and survive the degradation procedure. Thus, it seems relevant to determine whether a complex nervous system organization (e.g., cortical storage of memories) is required for such memory flexibility. Extinction and latent inhibition have been obtained with several invertebrate species that have a nervous system with a relatively simple organization, such as the honeybee (Apis mellifera; see, e.g., Bitterman, Menzel, Fietz, & Schäfer, 1983; Takeda, 1961), the common fruit fly (Drosophila melanogaster; Beck, Schroeder, & Davis, 2000; Tully & Quinn, 1985), the rusty crayfish (Orconectes rusticus; Acquistapace, Hazlett, & Gherardi, 2003; Nathaniel, Panksepp, & Huber, 2009), and neohelice crabs (Chasmagnatus granulata; Pedreira, Dimant, Tomsic, Queseda-Allue, & Maldonado, 1995; Tomsic, Pedreira, Romano, Hermitte, & Maldonado, 1998) (see the General discussion for further examples of contingency degradation in invertebrates). The present research investigates acquisition, extinction, and spontaneous recovery of odor aversions in the common garden slug, Lehmannia valentiana. Other pulmonate mollusks, such as leopard slugs (Limax maximus) and land snails (Helix aspersa), exhibit rapid acquisition of taste aversions (Sahley, Gelperin, & Rudy, 1981) and preferences (Loy, Fernández, & Acebes, 2006), as well as a variety of learning effects such as differential and trace conditioning (Sahley, Martin, & Gelperin, 1990), blocking (Acebes, Solar, Carnero, & Loy, 2009; Sahley, Rudy, & Gelperin, 1981), second-order conditioning (Loy et al., 2006), and sensory preconditioning (Kojima et al., 1998; Loy et al., 2006; Suzuki, Sekiguchi, Yamada, & Mizukami, 1994). Contingency degradation through extinction (Limax maximus; Sahley et al., 1990) and latent inhibition (Helix aspersa; Loy et al., 2006) and spontaneous recovery from extinction (Helix aspersa; Álvarez, Morís, Luque, & Loy, 2014) have been reported in terrestrial mollusks using appetitive learning preparations in which an odor was paired with access to food. However, there are no reports in the literature of recovery from latent inhibition in these species, and no reports of recovery from extinction using an aversive preparation.
The experiments reported here use an approach–withdrawal measure, in which freely moving animals are presented with a food odor previously paired with an aversive US. Experiment 1 assessed latent inhibition (CS preexposure) and spontaneous recovery from latent inhibition using a between-groups design. Experiment 2 assessed extinction (CS postexposure) and spontaneous recovery from extinction using a within-subjects design, which allowed for assessment of stimulus specificity of the contingency degradation procedures.
Experiment 1
Experiment 1 investigated the occurrence of latent inhibition and spontaneous recovery from latent inhibition in the garden slug, Lehmannia valentiana. Subjects in the latent inhibition (LI) condition received repeated exposures to CS A, whereas subjects in the control condition received equivalent exposure to the apparatus. Then, all subjects received one CS A–US pairing. Conditioned approach/avoidance responses were assessed 1 day after conditioning to determine whether CS preexposure reduced conditioned responding (latent inhibition test). Five days later, responding was reassessed to determine whether spontaneous recovery of the conditioned response had occurred.
Method
Subjects
The subjects were 16 experimentally naive adult garden slugs (Lehmannia valentiana, 0.7–2.1 g), captured in a local garden and randomly assigned to the LI and control groups (ns = 8). Subjects were individually housed in plastic containers (116 mm in diameter, 62 mm in depth) lined with moistened paper towels and covered with perforated lids, placed in a vivarium maintained on an 8 h/16 h light/dark cycle. Access to food was restricted to 0.14 ml of their maintenance feed (moistened rat chow enriched with calcium carbonate and reptile vitamins [ReptiviteTM brand] in a 1:0.2:0.4 proportion) offered in the evening of every third day (leftover food was removed the subsequent morning). Experimental manipulations were conducted during the dark portion of the cycle, under a red light.
Procedure
Stimuli
CS A was cucumber extract (400 ml of pure fruit juice diluted in 250 ml of tap water), quickly frozen for storage at −20 °C, and thawed immediately before use. The US was a 0.4 % (w/v) solution of quinidine sulfate, diluted in slug saline (70 mM NaCl, 2 mM KCl, 4.7 mM MgCl2, 4.9 mM CaCl2, 5 mM glucose, and 5.0 mM HEPES; pH adjusted to 7.45 with NaOH).
Preexposure
On Days 1–3, all subjects in the LI group received five daily 5-min exposures to CS A with an intertrial interval (ITI) of 10 min. Two ml of the CS were applied to a filter paper lining the bottom of a dish (90 mm in diameter, 20 mm in depth), topped with a 1.5-mm-thick grid floor. Subjects were placed atop the grid and the dish was covered with a lid. All subjects in the control group received equivalent treatment, except that their apparatus lacked the filter paper and stimulus.
Conditioning
On Day 4, subjects received one pairing of CS A and the US. Subjects were exposed to the CS for 3.5 min, as described for the preexposure phase, and then transferred to a second dish lined with filter paper soaked with 1.5 ml of the CS. (Placing the subjects on a CS-lined plate was intended to reduce potential differences between the contexts of preexposure and conditioning, which are known to profoundly disrupt the CS preexposure effect; see, e.g., Channell & Hall, 1983.) Immediately after a subject was placed inside the dish, 1.5 ml of the US were added in front of the head of the subject. The US produced a strong unconditioned response (retraction of the posterior antenna, head stretching and raising, writhing, and copious mucus secretion). If a subject climbed onto the walls or lid of the US container, it was placed back in the middle of the dish. After 1.5 min of exposure to the US, subjects were washed for 15 s and returned to their housing containers. The specific procedure and parameters (US concentration and time of exposure to the US) were derived from pilot research that indicated they were ideal for obtaining intermediate levels of conditioning, which would help to avoid ceiling effects that could obscure the effects of the LI treatment.
Assessment of latent inhibition and spontaneous recovery
On Days 5 and 10, responding and spontaneous recovery of conditioned responding to CS A, respectively, were assessed by determining the subjects’ willingness to approach the CS. Subjects were placed on the center of a 250 × 200 mm polycarbonate surface and allowed to move in the direction of their choice. After a minimum of 20 mm of continuous movement in the same direction, a 9-cm-diameter, 1-cm-wide, 180-degree filter paper was placed in front of the subject, with the center of this semicircle placed approximately 40 mm in front of the head (Fig. 1). The stimulus solution (1 ml) was applied to the filter paper (procedure adapted from Matsuo, Hitomi, Watanabe, & Kirino, 2002). Subjects that took longer than 120 s to start moving were prompted to do so by the experimenter gently squeezing their tail with blunt tweezers. Behavior was monitored for 180 s following presentation of the CS. Subjects were considered to be approaching the stimulus if their orientation vector crossed the stimulus area. (This vector was created by drawing an imaginary line through the middle of the head and antenna; see Fig. 1.) Subjects crossing over the stimulus area were considered to be approaching the stimulus unless the top of their heads was more than 25 mm past the stimulus area (see Fig. 1). After each test trial, subjects were thoroughly rinsed for 15 s with distilled water and returned to their housing containers.
Graphic description of the experimental procedure: When the subject had moved continuously for a minimum of 20 mm, the stimulus area (a 10-mm-thick, 90-mm-diameter half-circle) was placed approximately 40 mm in front of the head of the subject, and 1.0 ml of the CS solution was applied to the stimulus area. Subjects were considered to be approaching the stimulus area if the head orientation vector crossed the stimulus area, or if the subject’s head was located inside a 25-mm perimeter outside the stimulus area after crossing the stimulus area
Data scoring
One rater scored approach times as the animals were being tested. A second rater, who was blind to the procedure, scored videos of the test trials. The two scores were then averaged. All approach scores were converted to percentage of total test time (180 s). An alpha level of .05 was adopted for all statistical analyses. Effect sizes for all F tests were quantified with the partial eta squared statistic (ηp 2), and all mean comparisons were quantified with both Pearson’s r and Cohen’s d statistics.
Results and discussion
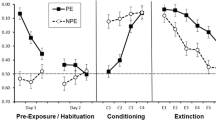
Figure 2 presents the data collected in Experiment 1. In the LI test (Day 5), more approach was observed in the LI group, which received preexposure to the CS, than in the control group [F(1, 14) = 16.80, MSE = 729.30, ηp 2 = 0.55]. That is, degradation of the CS–US contingency through preexposure attenuated conditioned responding. Importantly, spontaneous recovery of the withdrawal response was observed 5 days later (Day 10), with equivalent responding in the two groups regardless of whether or not preexposure had taken place [F(1, 14) < 1.0]. Indeed, subjects exhibited less approach in the spontaneous recovery test than in the LI test for group LI [t(8) = 2.61, r = 0.74, d = 2.19], but approach times remained the same for the control group [t(8) = 0.41].
Results of Experiment 1: Subjects in the LI condition received preexposure to the CS (cucumber) and then received CS–US pairings. Subjects in the control condition did not receive preexposure. The LI test occurred 24 h after conditioning. The spontaneous recovery test occurred 5 days later. Brackets represent the standard error of the mean
CS preexposure reduced slugs’ expression of aversion to an odor paired with a noxious US. However, this reduced aversion was temporary, and was observed during a subsequent spontaneous recovery test. Thus, the degradation procedure did not prevent the acquisition or the retrieval of the CS–US association.
Experiment 2
In Experiment 1, exposure to a CS before conditioning (CS–US pairings) reduced responding to the CS, and a time lapse imposed between conditioning and testing resulted in spontaneous recovery of the conditioned response. Experiment 2 attempted to provide evidence of another contingency degradation procedure—namely, extinction (CS postexposure). Experiment 2 used a similar design to the one used in Experiment 1, except that two food odors, CSs A and B, were paired with an aversive US. Then, one of the stimuli, CS A, was presented alone (extinction). Extinction was assessed by comparing responding to extinguished CS A and responding to nonextinguished CS B. The use of a within-subjects design allowed for assessment of the stimulus specificity of contingency degradation (i.e., we determined whether degrading the contingency for one CS–US association affected other CS–US associations). Ten days after extinction, we again assessed responding to CSs A and B to determine retention of the CS B–US association and spontaneous recovery of the CS A–US association.
Method
Subjects
The subjects were 12 experimentally naive garden slugs (Lehmannia valentiana). The experiment was completed in two replications (n = 8 for Replication 1 and n = 4 for Replication 2). All subjects were adults (1.4–1.9 g) captured in a local garden, and were housed and maintained as described in Experiment 1.
Procedure
Stimuli
CSs A and B were cucumber and tomato extracts, prepared and stored as described for Experiment 1. The US was either a 0.8 % (w/v) suspension of quinine sulfate (Replication 1) or a 0.8 % (w/v) solution of quinidine sulfate (Replication 2). Both salts were diluted in slug saline, as described in Experiment 1. Quinine and quinidine are stereoisomers with similar pharmacokinetic properties, and no differences were expected or observed in their effectiveness as USs (the use of one vs. the other compound was a result of availability). Thus, the data from both replications were pooled.
Conditioning
On Day 1, subjects received two pairings of either CS A or CS B (tomato or cucumber, counterbalanced) and the US. Two ml of the CS were applied to a filter paper that lined the bottom of a dish (90 mm in diameter, 20 mm in depth), topped with a 1.5-mm-thick grid floor. Subjects were placed atop the grid and the dish was covered with a lid. After 2 min, subjects were transferred to an identical dish lined with filter paper soaked with 2 ml of the US, and placed directly atop the US-soaked filter paper. A further 2 ml of the US were added in front of the subject’s head. This form of US exposure resulted in more robust conditioning than was used in Experiment 1, which was desirable to assess extinction without risking a floor effect. After 2 min of exposure to the US, subjects were thoroughly washed for 15 s with distilled water and returned to their housing containers. Two hours later, the procedure was repeated to complete the second trial with the CS. On Day 2, subjects received conditioning with the other CS, using the same procedure as on Day 1. The order of conditioning (A in Day 1 and B in Day 2, or vice versa) was counterbalanced.
Extinction training and assessment
On Days 3–5, subjects received 15 daily, 2-min exposures to CS A alone, as described for Day 1, with an ITI of 10 min. On Day 5, 30 min after the last extinction trial, subjects were tested for responding to either CS A or CS B (order counterbalanced) and, 30 min later, they were tested with the other CS. The test procedure was identical to that described for Experiment 1.
Assessment of spontaneous recovery
On Day 15, maintenance of responding to CS B and spontaneous recovery of conditioned responding to CS A were assessed using the test procedure described above (order of testing was counterbalanced).
Two subjects in Replication 1 died during the experiment and their data were excluded from all analyses.
Results and discussion
Figure 3 presents the data collected in Experiment 2. Extinction was analyzed using a 2 (Stimulus: CS A vs. CS B) × 2 (Replication) analysis of variance (ANOVA), which yielded a main effect of stimulus [F(1, 8) = 8.72, MSE = 645.31, ηp 2 = 0.52], reflecting more approach to CS A than to CS B. Thus, extinction was specific to the stimulus that received CS postexposure. Spontaneous recovery was analyzed using a similar 2 (Stimulus) × 2 (Replication) ANOVA. This analysis did not reveal a main effect of stimulus [F(1, 8) < 1.0], suggesting that responding to CSs A and B was similar after the retention interval. Indeed, responding was lower in the spontaneous recovery test than in the extinction test for CS A [t(9) = 2.60, r = 0.66, d = 1.73], but not for CS B [t(9) = 1.42]. In all analyses, neither the main effect of replication nor the interactions of this and any other factors were significant [largest F(1, 8) = 1.09].
Results of Experiment 2: All subjects were exposed to both CS A and CS B (cucumber and tomato, counterbalanced), which were paired with the US. CS A received extinction, whereas CS B did not. The spontaneous recovery test occurred 10 days after extinction. Brackets represent the standard error of the mean
With the present preparation, garden slugs withdrew from an odor paired with an aversive US (CS B). However, after repeated postconditioning exposure to similarly trained CS A, subjects approached this odor; that is, the aversion underwent extinction. Importantly, exposure to CS A had an effect on CS A only; subjects continued to avoid nonextinguished CS B. Furthermore, avoidance of CS A was observed again after a retention interval (spontaneous recovery), suggesting that the association survived the contingency degradation procedure.
General discussion
The present studies provide evidence of attenuated responding following two contingency-degrading procedures, namely CS pre- and postexposure (latent inhibition and extinction, respectively) in the garden slug, Lehmannia valentiana. The impact of both degradation procedures was temporary, with conditioned responding returning to robust levels following a time lapse. That is, the memory of the aversive conditioning event remained intact despite contingency degradation, and for several days after conditioning. The relatively equivalent effect of the CS pre- and postexposure manipulations suggest that contingency degradation can affect behavior based on both previously and subsequently acquired associations, but that neither procedure eliminates the CS–US association. Furthermore, the observation of spontaneous recovery after CS pre- and postexposure suggests that, even in an animal with a relatively simple neural organization, memories of events with high biological relevance are not “erased” by degradation treatments. To investigate learning and memory in land mollusks, we present a novel preparation that uses both an aversive US and a choice response to record behavior. We also present evidence of spontaneous recovery of the conditioned response following a time lapse after contingency degradation.
Contingency degradation of aversive CS–US associations through extinction has been linked to specific brain circuits and, specifically, to cortical areas and subcortical areas involved in emotion regulation (e.g., the medial prefrontal cortex, amygdala, and hippocampus; see Sotres-Bayon, Cain, & LeDoux, 2006, for a review). Latent inhibition has been associated to cortical and subcortical areas related to behavioral flexibility and sensitivity to reward (e.g., the entorhinal cortex and nucleus accumbens; see Weiner, 2010, for a review). However, contingency degradation effects have been reported in several species that lack a complex cortical organization. For example, extinction has been successfully observed in several species of the phylum Arthropoda. In the subphylum Hexapoda, extinction has been reported for two class Insecta species, the honeybee (Apis mellifera; Bitterman, Menzel, Fietz, & Schäfer, 1983; Sandoz & Pham-Delègue, 2004; Stollhoff, Menzel, & Eisenhardt, 2005; Takeda, 1961) and the common fruit fly (Drosophila melanogaster; Lagasse, Devaud, & Mery, 2009; Qin & Dubnau, 2010; Schwaerzel, Heisenberg, & Zara, 2002; Tully & Quinn, 1985). In the subphylum Crustacea, extinction has been reported for two species in class Malocostraca, the rusty crayfish (Orconectes rusticus; Nathaniel et al., 2009) and the neohelice crab (Chasmagnatus granulata; Tomsic et al., 1998). In the phylum Mollusca, in which most species (with exception of the cephalopods) lack a brain and instead exhibit a series of paired ganglia that control all sensory and motor functions, extinction has been most widely studied in the aquatic species Aplysia californica (see, e.g., Colwill, Goodrum, & Martin, 1997), with just two reports of extinction in land mollusks, the leopard slug (Limax maximus; Sahley et al., 1990) and the garden snail (Helix aspersa; Álvarez et al., 2014).
Contingency degradation via latent inhibition has also been reported in two Arthropoda species of the subphylum Hexapoda, the honeybee (Apis mellifera; Abramson & Bitterman, 1986; Bitterman et al., 1983; Chandra, Hosler, & Smith, 2000; Chandra, Hunt, Cobey, & Smith, 2001; Ferguson, Cobey, & Smith, 2001; Fernández, Giurfa, Devaud, & Farina, 2012) and the common fruit fly (Drosophila melanogaster; Beck et al., 2000). In the subphylum Crustacea, latent inhibition has been reported in neohelice crabs (Chasmagnatus granulata; Pedreira et al., 1995; Tomsic et al., 1998) and the rusty and Northern crayfish (Orconectes rusticus and Orconectes virilis; Acquistapace et al., 2003). In the phylum Nematoda, latent inhibition has been reported in the roundworm, Caenorhabditis elegans (Rankin, 2000). Finally, in the phylum Mollusca, latent inhibition has been reported in the garden snail (Helix aspersa), using an appetitive conditioning preparation (Loy et al., 2006). Surprisingly, despite the relatively large number of reports of extinction in aquatic mollusks, such as Aplysia californica, latent inhibition has not been obtained in these species, even after a large number of preexposure trials (Farley, 1987).
Spontaneous recovery from extinction has been reported in invertebrate species, including the honeybee (Apis mellifera; Bitterman et al., 1983; Sandoz & Pham-Delègue, 2004; Stollhoff et al., 2005; Takeda, 1961), the common fruit fly (Drosophila melanogaster; Engel & Wu, 1996), neohelice crabs (Chasmagnatus granulata; Hepp, Pérez-Cuesta, Maldonado, & Pedreira, 2010; Merlo & Romano, 2008), the pond snail (Lymnaea stagnalis; Sangha, Scheibenstock, Morrow, & Lukowiak, 2003; but see Richards, Farley, & Alkon, 1984), and the garden snail (Helix aspersa; Álvarez et al., 2014). Spontaneous recovery from latent inhibition has not been previously reported in invertebrates.
The use of invertebrate species for the study of learning has many advantages, including an understanding of the generality of basic learning phenomena, and the development of models for the cellular basis of learning and memory (see, e.g., Burrell & Sahley, 2001). Aquatic mollusks, such as species of the Aplysia genus (e.g., Aplysia californica) and Hermissenda crassicornis have long been used to study the cellular basis of learning and memory (Carew, Walters, & Kandel, 1981; Farley, 1987). The procedures used with these species are well established and can be applied to a multitude of experimental designs. However, they require that the subject be restrained, which limits the applicability of the procedure to passive exposure to a stimulus and a subject’s unconditioned and conditioned reflexive reactions to it. The present studies used freely moving pulmonate mollusks in an aversive setting that can be viewed as analogous to conditioned taste aversion and approach–withdrawal procedures in vertebrates. The present aversive preparation provides multiple advantages over the use of other preparations that use appetitively motivated behavior or small reflexive responses to assess learning (see, e.g., Loy et al., 2006). Strong aversions can be conditioned with as little as one trial, and memory of this one-trial learning can last for up to 25 days (see, e.g., Gelperin, 1975). However, unpublished pilot data from our laboratory suggests that learning rate can be manipulated by changing the concentration of the US or the time of exposure to the US. Another advantage of using animals that phylogenetically diverge from the phylum Chordata, frequently used in learning and memory research, is that it allows for a clear determination of basic phenomena that are common across multiple taxonomic branches. Observing acquisition, discrimination, extinction, and latent inhibition in mollusks suggests that an organized brain structure such as that found in Chordata is not fundamental to observe these phenomena, but that they may occur in a subset of organized cells or even at the cellular level.
References
Abramson, C. I., & Bitterman, M. E. (1986). Latent inhibition in honeybees. Animal Learning & Behavior, 14, 184–189. doi:10.3758/BF03200054
Acebes, F., Solar, P., Carnero, S., & Loy, I. (2009). Blocking of conditioning of tentacle lowering in the snail (Helix aspersa). Quarterly Journal of Experimental Psychology, 62, 1315–1327. doi:10.1080/17470210802483545
Acquistapace, P., Hazlett, B. A., & Gherardi, F. (2003). Unsuccessful predation and learning of predator cues by crayfish. Journal of Crustacean Biology, 23, 364–370. doi:10.1163/20021975-99990346
Álvarez, B., Morís, J., Luque, D., & Loy, I. (2014). Extinction, spontaneous recovery and reinstatement in the garden snail, Helix aspersa. Animal Behaviour, 92, 75–83. doi:10.1016/j.anbehav.2014.03.023
Beck, C. D. O., Schroeder, B., & Davis, R. L. (2000). Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. Journal of Neuroscience, 20, 2944–2953.
Bitterman, M. E., Menzel, R., Fietz, A., & Schäfer, S. (1983). Classical conditioning of proboscis extension in honeybees. Journal of Comparative Psychology, 97, 107–119. doi:10.1037/0735-7036.97.2.107
Bouton, M. E. (1993). Context, time, and memory retrieval in the interference paradigms. Psychological Bulletin, 114, 80–99. doi:10.1037/0033-2909.114.1.80
Burrell, B. D., & Sahley, C. L. (2001). Learning in simple systems. Current Opinion in Neurobiology, 11, 757–764. doi:10.1016/S0959-4388(01)00281-1
Carew, T. J., Walters, E. T., & Kandel, E. R. (1981). Classical conditioning in a simple withdrawal reflex in Aplysia californica. The Journal of Neuroscience, 1, 1426–1437.
Chandra, S. B. C., Hosler, J. S., & Smith, B. H. (2000). Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera). Journal of Comparative Psychology, 114, 73–85. doi:10.1037/0735-7036.114.1.86
Chandra, S. B. C., Hunt, G. J., Cobey, S., & Smith, B. H. (2001). Quantitative trait loci associated with reversal learning and latent inhibition in honeybees (Apis mellifera). Behavior Genetics, 31, 275–285. doi:10.1023/A:1012227308783
Channell, S., & Hall, G. (1983). Contextual effects in latent inhibition with an appetitive conditioning procedure. Animal Learning & Behavior, 11, 67–74. doi:10.3758/BF03212309
Colwill, R. M., Goodrum, K., & Martin, A. (1997). Pavlovian appetitive discriminative conditioning in Aplysia californica. Animal Learning & Behavior, 25, 268–276. doi:10.3758/BF03199084
Engel, J. E., & Wu, C.-F. (1996). Altered habituation of an identified escape circuit in Drosophila memory mutants. Journal of Neuroscience, 16, 3486–3499. doi:10.1126/science.1670967
Escobar, M., Arcediano, F., & Miller, R. R. (2002). Latent inhibition and contextual associations. Journal of Experimental Psychology: Animal Behavior Processes, 28, 123–136. doi:10.1037//0097-7403.28.2.123
Farley, J. (1987). Contingency learning and causal detection in Hermissenda: I. Behavior. Behavioral Neuroscience, 101, 13–27. doi:10.1037/0735-7044.101.1.13
Ferguson, H. J., Cobey, S., & Smith, B. H. (2001). Sensitivity to a change in reward is heritable in the honeybee, Apis mellifera. Animal Behaviour, 61, 527–534. doi:10.1006/anbe.2000.1635
Fernández, V. M., Giurfa, M., Devaud, J.-M., & Farina, W. M. (2012). Latent inhibition in an insect: The role of aminergic signaling. Learning & Memory, 19, 593–597. doi:10.1101/lm.028167.112
Gelperin, A. (1975). Rapid food-aversion learning by a terrestrial mollusk. Science, 189, 567–570. doi:10.1126/science.1145215
Hepp, Y., Pérez-Cuesta, L. M., Maldonado, H., & Pedreira, M. E. (2010). Extinction memory in the crab Chasmagnathus: Recovery protocols and effects of multitrial extinction training. Animal Cognition, 13, 391–403. doi:10.1007/S10071-009-0288-Y
Hollis, K. L. (1997). Contemporary research on Pavlovian conditioning: A “new” functional analysis. American Psychologist, 52, 956–965. doi:10.1037/0003-066X.52.9.956
Kojima, S., Kobayashi, S., Yamanaka, M., Sadamoto, H., Nakamura, H., Fujito, Y., … Ito, E. (1998). Sensory preconditioning for feeding response in the pond snail, Lymnaea stagnalis. Brain Research, 808, 113–115. doi:10.1016/S0006-8993(98)00823-3
Kraemer, P. J., & Spear, N. E. (1992). The effect of nonreinforced stimulus exposure on the strength of a conditioned taste aversion as a function of retention interval: Do latent inhibition and extinction involve a shared process? Animal Learning & Behavior, 20, 1–7. doi:10.3758/BF03199940
Lagasse, F., Devaud, J.-M., & Mery, F. (2009). A switch from cycloheximide-resistant consolidated memory to cycloheximide-sensitive reconsolidation and extinction in Drosophila. Journal of Neuroscience, 29, 2225–2230. doi:10.1523/JNEUROSCI.3789-08.2009
Loy, I., Fernández, V., & Acebes, F. (2006). Conditioning of tentacle lowering in the snail (Helix aspersa): Acquisition, latent inhibition, overshadowing, second-order conditioning, and sensory preconditioning. Learning & Behavior, 34, 305–314. doi:10.3758/BF03192885
Lubow, R. E., & Moore, A. U. (1959). Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology, 52, 415–419. doi:10.1037/h0046700
Matsuo, R., Hitomi, T., Watanabe, S., & Kirino, Y. (2002). Delayed-onset amnesia caused by protein synthesis inhibition in odor–taste associative memory of the terrestrial slug Limax valentianus. Neuroscience Letters, 334, 201–205. doi:10.1016/S0304-3940(02)01089-3
Merlo, E., & Romano, A. (2008). Memory extinction entails the inhibition of the transcription factor NF-kB. PLoS One, 3, e3687. doi:10.1371/journal.pone.0003687
Nathaniel, T. I., Panksepp, J., & Huber, R. (2009). Drug-seeking behavior in an invertebrate system: Evidence of morphine-induced reward, extinction and reinstatement in crayfish. Behavioural Brain Research, 197, 331–338. doi:10.1016/j.bbr.2008.08.043
Pavlov, I. P. (1927). Conditioned reflexes. London: Oxford University Press.
Pedreira, M. E., Dimant, B., Tomsic, D., Queseda-Allue, L. A., & Maldonado, H. (1995). Cycloheximide inhibits context memory and long-term habituation in the crab Chasmagnathus. Pharmacology, Biochemistry, & Behavior, 52, 385–395. doi:10.1016/0091-3057(95)00124-F
Pineño, O., & Miller, R. R. (2005). Primacy and recency effects in extinction and latent inhibition: A selective review with implications for models of learning. Behavioural Processes, 69, 223–235. doi:10.1016/j.beproc.2005.02.006
Qin, H., & Dubnau, J. (2010). Genetic disruptions of Drosophila Pavlovian learning leave extinction learning intact. Genes, Brain and Behavior, 9, 203–212. doi:10.1111/j.1601-183X.2009.00548.x
Rankin, C. H. (2000). Context conditioning in habituation in the nematode Caenorhabditis elegans. Behavioral Neuroscience, 114, 496–505. doi:10.1037/0735-7044.114.3.496
Rescorla, R. A. (1967). Pavlovian conditioning and its proper control procedures. Psychological Review, 74(1), 71–80. doi:10.1037/h0024109
Rescorla, R. A. (1988). Pavlovian conditioning: It’s not what you think it is. American Psychologist, 53, 151–160. doi:10.1037/0003-066X.43.3.151
Rescorla, R. A. (2001). Retraining of extinguished Pavlovian stimuli. Journal of Experimental Psychology: Animal Behavior Processes, 27, 115–124. doi:10.1037/0097-7403.27.2.115
Rescorla, R. A. (2004). Spontaneous recovery. Learning & Memory, 11, 501–509. doi:10.1101/lm.77504
Richards, W. G., Farley, J., & Alkon, D. L. (1984). Extinction of associative learning in Hermissenda: Behavior and neural correlates. Behavioural Brain Research, 14, 161–170. doi:10.1016/0166-4328(84)90185-2
Sahley, C. L., Gelperin, A., & Rudy, J. W. (1981a). One-trial associative learning modifies food odor preferences of a terrestrial mollusc. Proceedings of the National Academy of Sciences, 78, 640–642. doi:10.1073/pnas.78.1.640
Sahley, C. L., Martin, K. A., & Gelperin, A. (1990). Analysis of associative learning in the terrestrial mollusc Limax maximus. II. Appetitive learning. Journal of Comparative Physiology, 167, 339–345. doi:10.1007/bf00192569
Sahley, C. L., Rudy, J. W., & Gelperin, A. (1981b). An analysis of associative learning in a terrestrial mollusc: Higher-order conditioning, blocking, and a transient US pre-exposure effect. Journal of Comparative Physiology, 144, 1–8.
Sandoz, J.-C., & Pham-Delègue, M.-H. (2004). Spontaneous recovery after extinction of the conditioned proboscis extension response in the honeybee. Learning & Memory, 11, 586–597. doi:10.1101/lm.81504
Sangha, S., Scheibenstock, A., Morrow, R., & Lukowiak, K. (2003). Extinction requires new RNA and protein synthesis and the soma of the cell right pedal dorsal 1 in Lymnaea stagnalis. The Journal of Neuroscience, 23, 9842–9851.
Schwaerzel, M., Heisenberg, M., & Zara, T. (2002). Extinction antagonizes olfactory memory at subcellular level. Neuron, 35, 951–960. doi:10.1016/S0896-6273(02)00832-2
Sotres-Bayon, F., Cain, C. K., & LeDoux, J. (2006). Brain mechanisms of fear extinction: Historical perspectives on the contribution of the prefrontal cortex. Biological Psychiatry, 60, 329–336. doi:10.1016/j.biopsych.2005.10.012
Stollhoff, N., Menzel, R., & Eisenhardt, D. (2005). Spontaneous recovery from extinction depends on the reconsolidation of the acquisition memory in an appetitive learning paradigm in the honeybee (Apis mellifera). Journal of Neuroscience, 25, 4485–4492. doi:10.1523/jneurosci.0117-05.2005
Suzuki, H., Sekiguchi, T., Yamada, A., & Mizukami, A. (1994). Sensory preconditioning in the terrestrial mollusk, Limax flavus. Zoological Science, 11, 121–125.
Takeda, K. (1961). Classical conditioned response in the honey bee. Journal of Insect Physiology, 6, 143–173. doi:10.1016/0022-1910(61)90060-9
Tomsic, D., Pedreira, M. E., Romano, A., Hermitte, G., & Maldonado, H. (1998). Context–US association as a determinant of long-term habituation in the crab Chasmagnathus. Animal Learning & Behavior, 26, 196–209. doi:10.3758/BF03199212
Tully, T., & Quinn, W. G. (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology, 157, 263–277. doi:10.1007/BF01350033
Weiner, I. (2010). What the brain teaches us about latent inhibition (LI): The neural substrates of the expression and prevention of LI. In R. E. Lubow & I. Weiner (Eds.), Latent inhibition: Cognition, neuroscience, and applications to schizophrenia (pp. 372–415). Cambridge: Cambridge University Press.
Author note
This research was partially funded by an Auburn University Undergraduate Research Fellowship to K.G., who is now at the University of Alabama Medical School. We thank Kristen Haynes for assistance with this project, and Duncan Amegbletor, Kristen Haynes, and R. Alexander Sauer for comments on an early version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escobar, M., Dunaway, E.P. & Gennaro, K.H. Conditioned avoidance responses survive contingency degradation in the garden slug, Lehmannia valentiana . Learn Behav 42, 305–312 (2014). https://doi.org/10.3758/s13420-014-0147-9
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-014-0147-9