Abstract
It is unknown whether the manner with which an item is encoded in isolation, immediately before it is encoded into an inter-inter association, influences associative memory. We therefore presented the items of to-be-encoded associative pairings sequentially and manipulated how each first item of a pair was encoded (before associative encoding could begin). Furthermore, we recorded ERPs during memory encoding to investigate the neurocognitive processes that might relate pre-associative item encoding to subsequent associative memory performance. Behaviorally, we found that pre-associative item elaboration (vs. no elaboration) led to a memory tradeoff—enhanced item memory relative to impaired associative memory. This tradeoff likely reflected that item elaboration reduced cognitive resources for ensuing associative encoding, indexed by a reduced P300 and frontal slow wave at the time of associative encoding. However, frontal slow wave subsequent memory effects measured during pre-associative item encoding revealed that, for a given item, greater semantic elaboration was related to better item and associative memory while greater visual elaboration was related to better item and worse associative memory. Thus, there are likely two opposing ways in which pre-associative item encoding can influence associative memory: (1) by depleting encoding resources to impair associative memory and (2) by scaffolding inter-item associations to enhance associative memory. When item encoding occurs immediately before associative encoding, it appears that the temporary depletion of encoding resources is more important in determining later memory performance. Future research should compare the independent effects of resource depletion and encoding strategy during pre-associative item encoding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Memory for previously encountered objects or items without memory for the relationships between these items often is insufficient for guiding behavior. For example, vivid memories of the faces and names of new colleagues, without remembering which names are paired with which faces, would not allow us to address each colleague correctly. Unfortunately, this aspect of memory, referred to as associative memory, is typically more difficult and more likely to be impacted by factors such as divided attention, aging, and psychiatric impairment (Castel & Craik, 2003; Gold et al., 2006; Old & Naveh-Benjamin, 2008). To investigate the processes that affect our ability to encode information associatively, researchers typically present multiple items simultaneously while manipulating and/or measuring potentially relevant encoding factors (e.g., encoding strategy, attentional demands, brain activity, etc.) and then examine how well participants are later able to remember the associations. Although this approach has led to important insights, in this design, the encoding of associative information occurs simultaneously with the encoding of item information, so it is difficult to disentangle how item encoding processes independently affect associative memory. Furthermore, given that there often are temporal gaps between the items that we encounter in our daily life (e.g., seeing a new colleague’s face before hearing her name), a potentially important question is: Does the manner with which an item is encoded alone, before it is bound into an inter-item associative memory, affect associative memory for the pairing? The present study thus examines such “pre-associative item encoding” processes.
Encoding ERPs and subsequent memory effects
Event-related potentials (ERPs) measured at the time of encoding can provide unique insight into memory mechanisms (Kronesisen et al., in press). Of particular relevance is an ERP component called the frontal slow wave, which is a sustained voltage deflection that is typically maximal at frontal electrodes and is elicited by tasks that require controlled or associative processing (Bosch et al., 2001; Kamp et al., 2016). Importantly, the amplitude of the frontal slow wave typically increases with increasing associative processing during memory encoding, whether this involves elaborating upon the relationship between multiple items or elaborating upon a single item and its relation to prior knowledge or memories (Fabiani et al., 1990; Forester, Kroneisen, et al., 2020b, 2020c; Kamp et al., 2017). The size of the frontal slow wave at encoding also often varies with trial-to-trial variability in memory retrieval successes, a phenomenon referred to as a “subsequent memory effect” (SME; Karis et al., 1984; Paller & Wagner, 2002). For example, if ERPs elicited during the encoding of pairs of words are back sorted based on whether the pairings are subsequently remembered or forgotten on a memory test, a frontal slow wave SME often is observed, such that the frontal slow wave at encoding is more positive for subsequently remembered pairs (Forester, Halbeisen, et al., 2020; Kamp et al., 2017). The frontal slow wave therefore provides a window into the degree of higher-level elaborative or associative processes that occur during memory encoding, as well the relationship between these processes and later memory performance for a given item or pairing. Another ERP component for examining memory encoding is the P300, which is observed as a positive peak at centroparietal electrodes that occurs before the frontal slow wave (Karis et al., 1984; Sutton et al., 1965). The size of the P300 increases with increased resource allocation during initial stimulus evaluation, whether due to salient stimulus attributes, motivational state, or resource capacity (Donchin, 1981; Hajcak & Foti, 2020; Johnson, 1986), and a P300 SME is commonly found when these lower-level factors are important in determining subsequent memory success (Johnson, 1995).
The P300 and frontal slow wave therefore provide distinct insight into the neurocognitive processes that are relevant to successful memory encoding and can be used to help disentangle the influence of item and associative encoding processes on associative memory. Indeed, in dissociating these ERP components, we recently found evidence hinting that individual differences in associative memory (specifically, age-related associative memory decline) may in part reflect differences in the manner with which the individual items of an associative pair are encoded, rather than differences in associative encoding processes per se (Kamp et al., 2022). Specifically, while the frontal slow wave during associative encoding was diminished in older adults (relative to younger adults), indicating impaired associative encoding, this associative frontal slow wave did not distinguish older adults with relatively strong associative memory from older adults with relatively weak associative memory. Instead, a reverse P300 SME for item memory was found in older adults with relatively weak associative memory, but not in those with strong associative memory. A reverse-item P300 SME—when a larger P300 is associated with worse subsequent item memory—may be reflective of especially superficial item encoding (Otten & Rugg, 2001), suggesting that differences in the manner of pre-associative item encoding might partially explain individual differences in associative memory among older adults. However, because we did not manipulate item encoding, and because we did not examine how neural activity during the encoding of an isolated item was related to associative memory for that item’s pairing, we did not test this idea directly. To our knowledge, such an influence of item encoding on associative memory has never been tested directly, even among healthy young adults. However, previous research can be used to guide predictions about how pre-associative item encoding might affect associative memory.
Impairment of associative memory through item encoding
First, a long line of research has shown that an item-specific encoding focus can limit the capacity for concurrent associative encoding, resulting in a tradeoff between item and associative memory (Hockley & Cristi, 1996). For example, when encoding two items simultaneously, creating a mental image of the items acting independently (item focus) leads to worse associative memory than creating a mental image of the items interacting (associative focus; Bower, 1970). This effect can also be seen when items are encountered sequentially, rather than simultaneously, as long as the item-specific focus is adopted in lieu of associative encoding. For example, Murray and Ranganath (2007) presented pairs of items sequentially and manipulated the focus of encoding at the time the second item was presented (and thus at the time that direct associative encoding became possible). Participants either judged whether the second item was living versus nonliving (item focus) or judged whether it could fit inside the previous item (associative focus). Worse associative memory was found in the item-focused encoding condition, further demonstrating that item-specific encoding can impair concurrent associative encoding.
Importantly, this line of research does not address how item-specific encoding processes affect associative memory when item encoding occurs before, and therefore not directly at the expense of, associative encoding. However, recent theoretical and empirical work indicates that memory encoding relies on a limited capacity resource, which when depleted, requires time to replenish (Popov & Reder, 2020). Thus, even without direct competition, an intensive pre-associative item focus could potentially impair associative memory by temporarily depleting the encoding resources needed for associative encoding.
Enhancement of associative memory through item encoding
Another long line of research suggests that aspects of item encoding can improve rather than impair associative memory. Specifically, some item features may provide a “scaffold” or “peg” upon which other items can be more easily integrated during inter-item associative processing, allowing for deeper or more elaborative associative encoding and enhanced associative memory (Craik & Tulving, 1975; Madan et al., 2010; Paivio, 1963). In a classic example, Paivio (1965) showed that associative memory for noun-adjective word pairings is enhanced when the noun is concrete rather than abstract, showing that intrinsic item features of a single item can influence inter-item associative memory. More recently, Liu et al. (2017) showed that when encoding arbitrary associations between faces and objects, if the face is known (i.e., a famous face), associative encoding fMRI activity is enhanced and this activity is related to improved associative memory performance. Similar effects also can be found by simply pre-exposing a subset of items to participants before the items are used in an associate memory task, with pairings that include at least one pre-exposed item leading to enhanced associative encoding neural activity and later memory retrieval (Dennis et al., 2015; Elbich et al., 2021; Kilb & Naveh-Benjamin, 2011; Reder et al., 2016). Thus, intrinsic item attributes, previous knowledge, and even previous familiarity for a single item can all improve associative memory. It therefore may be reasonable to speculate that, rather than resulting in a memory tradeoff, a pre-associative item focus that activates a rich semantic network, such as by elaborating on the meaning or conceptual relations of a single item, could have an enhancing effect on ensuing inter-item associative memory. This effect could occur either directly at the time of associative encoding by facilitating inter-item elaboration, during consolidation by accelerating neocortical representation, and/or during retrieval by providing more easily accessible retrieval cues (Bein et al., 2015; Craik & Tulving, 1975; van Kesteren et al., 2012).
Neurocognitive preparation for encoding
Although the above reviewed literature provides considerable, albeit indirect, evidence that pre-associative item encoding could influence associative memory, it offers relatively little insight into the precise neural encoding mechanisms that might underlie such an influence. However, a separate area of research may help to fill this gap. Specifically, recent research has shown that differences in neural activity occurring immediately before encoding may have an important effect on memory. In particular, ERP (Otten et al., 2006) and fMRI (Adcock et al., 2006) SMEs have been found in the seconds leading up to the presentation of a to-be-encoded item, suggesting that attentional or preparatory processes might play an important role in item and associative memory encoding, even before direct encoding can begin (Addante et al., 2015; Cohen et al., 2015; Madore & Wagner, 2022). Thus, just as these “pre-stimulus” SMEs provide insight into the preparatory processes that can influence memory, even before encoding has begun, pre-associative SMEs during item encoding may provide similar insight into the ways in which item encoding can influence associative memory, even before associative encoding has begun.
The present study
Putting these distinct lines of research together, it seems that an intensive pre-associative item focus could potentially impair associative memory by diverting resources away from associative memory encoding (either strategically or through depletion) or by limiting preparatory-specific processes. In contrast, an intensive pre-associative item focus could enhance associative memory if it serves a preparatory or faciliatory function, such as by providing scaffolding for inter-item integration. These predictions and their potential neural mechanisms, however, clearly require direct empirical testing.
In the present study, we recorded ERPs while presenting pairs of items sequentially in an associative memory-encoding task. Crucially, we manipulated how participants encoded the first item of a pair, before associative encoding could begin. A “Semantic” group was instructed to elaborate semantically upon the first item, a “Visual” group was instructed to elaborate upon its visual details, and a “Control” group was given no specific instructions. All groups used the same highly elaborative associative strategy (i.e., interactive imagery) to encode the pairing once the second item appeared.
With regard to behavioral memory performance, we tested whether intensive (i.e., Visual or Semantic) pre-associative item elaboration leads to a tradeoff between item and associative memory. To do so, we tested later recognition of the first item and its association, as well as the manner with which item and associative memories were retrieved by estimating familiarity and recollection parameters from receiver operating characteristics (ROC) curves (Yonelinas, 1994).
With regard to ERPs, the first question was whether neural activity elicited during the encoding of an item would correlate with subsequent item and associative memory. We hypothesized that the frontal slow wave during encoding of the first item would be more strongly correlated with subsequent item and associative memory in the Semantic group, relative to the Control group (enhanced item and associative SME), providing evidence that an increase in pre-associative semantic item elaboration supports associative memory. By contrast, we hypothesized that for the Visual group compared to the Control group, the frontal slow wave SME during pre-associative item encoding would be greater only for subsequent item (and not associative) memory, suggesting that item elaboration that is superficial in nature, although useful for item memory, does not support inter-item associations. In addition, we tested for these same patterns in the pre-associative item P300, to determine if lower-level resource allocation during pre-associative item encoding would be related to group differences in memory.
Finally, we asked how pre-associative item encoding would affect associative encoding, that is, once the second item was presented. Specifically, we tested whether the frontal slow wave to the second item of a pair as a measure of inter-item associative encoding (as well as encoding of the second item), and the P300 to the second item as a measure of cognitive resource allocation, as well as their respective SMEs, would be reduced in the Visual and Semantic groups.
Method
All procedures followed the ethical standards of the German Psychological Association and the Declaration of Helsinki and were approved in advance by the local ethics committee. All participants provided written informed consent.
Participants
A total of 90 participants (30 per group) took part in the study. Participants (70 females) were aged 19 to 38 years (M = 24.23) and were compensated with partial course credit or 9 Euros per hour.
Given N = 90 and a desired power of 0.8 (α = 0.05), we had sensitivity to detect medium sized interactions of f = 0.17 (Faul et al., 2007) between the between-subjects factor group (semantic vs. visual vs. control) and the within-subjects factor memory type (item vs. associative), as well as for within-subject tests, for behavioral memory performance. Because the ERP data from 11 participants were excluded (Section EEG recording), resulting in N = 79, we had the sensitivity to detect medium sized interactions and within-subject effects of f = 0.19 for the ERP analysis.
Stimuli
A total of 300 simple black line drawings and cliparts of common objects were used as stimuli. These stimuli were the same as those used by Kamp et al. (2022) and were originally obtained from the International Picture Naming Project (Szekely et al., 2004), from Kamp and Donchin (2015), and from online searches. The stimuli were preselected for their ease of identification based on ratings from an independent sample (for more details, see Kamp et al., 2022). The stimuli were presented on a gray background at a size of 8.5 cm at their longest dimension (width or height).
Encoding task
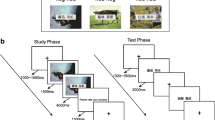
For each trial of the encoding task (Fig. 1), two items were presented sequentially, forming a pair. All participants were informed that their memory for the items individually, as well as for the pairing, would later be tested. Furthermore, all participants were identically instructed that when the first item disappeared and the second item appeared, they should form a mental image of the first item and the second item interacting (interactive imagery; Bower, 1970). For example, if a cookie (item 1) were followed by a rocket ship (item 2), participants might imagine the flames of the rocket ship toasting the cookie.
The only aspect of the task that differed between the groups was the instruction for how participants should focus on the first item, before the second item was presented, and therefore before item-item associative encoding could occur. For the Semantic group, participants were instructed to think of associations between the first item and other concepts or memories that came to mind. For example, if shown a cookie, they might think about how well the cookie would pair with a glass of milk or about the bakery where it might have been baked. For the Visual group, participants were instructed to focus on distinct visual features of the item in detail. For example, if the image of a cookie was shown, they might notice the difference in shade between the cookie and its chocolate chips or the texture of the crumbs that were falling to one side. For the Control group, participants were not given any specific instructions for how to focus on the first item before interactive imagery.
A total of 240 items, forming 120 pairs, were presented. The order and pairings of the items were randomized for each participant, with the restriction that the items of a pair could not be of the same semantic category (e.g., two animals could not be paired). Each trial began with a fixation cross for 2,000 ms, followed by the first item for 3,000 ms. The first item was then replaced by a fixation cross for 1,000 ms, before the second item was presented for 3,000 ms. After another 1,000-ms fixation cross, participants rated how well they were able to imagine the interaction between the two items on a scale of 1 (very well) to 4 (very poorly). After their response, the next trial began. There were a total of 120 trials in the task, and participants took self-paced breaks after every 40 trials.
Recognition task
The recognition task was identical for all groups (Fig. 1). Importantly, associative recognition was tested only if the item itself was first correctly recognized, ensuring that associative memory was unconflated with item memory for the first item. Each trial began with a fixation cross for 1,000 ms. To test item memory for item 1 images from the encoding task, for each trial, either an old item (an item 1 from the encoding task, two thirds of the trials) or a new item (a previously unseen item, one third of the trials) was presented. Participants responded by pressing the “1” key if they thought the item was old or the “2” key if they thought the item was new. Their response cleared the screen. After 500 ms, they were asked to rate how confident they were in their response between 1 (certain), 2 (somewhat certain), and 3 (uncertain). If the item was rated as “new,” the confidence rating response terminated the trial. If a participant rated the item as “old,” the confidence rating was followed by a fixation cross (1,000 ms). For the associative memory test, following the fixation cross, the first item was presented together with a second item. For two-thirds of the trials, the second item was same item 2 that had been presented with the first item during encoding (old pairing). For the remaining third of the trials, the second item came from a different pairing from the encoding task (recombined pairing). Whenever a new item had been incorrectly identified as old during the item memory test, it was paired with another novel item for the associative memory test to avoid providing feedback on the correctness of their item memory judgment. When both items were present on the screen, participants responded by pressing the “1” key if they thought the pairing was old or the “2” key if they thought the item was new (recombined). Their response cleared the screen. After 500 ms, they were asked to rate how confident they were in their response between 1 (certain), 2 (somewhat certain), and 3 (uncertain). Their response terminated the trial.
All 120 item-1 images were presented in the recognition task, as well as 60 new items, for a total of 180 trials. Participants took self-paced breaks after every 45 trials in the recognition task.
Procedure
After participants provided informed consent, they were fitted with an EEG cap, and the EEG recording was prepared. Participants then read on-screen instructions and completed practice trials for the encoding task. When the participant was ready to proceed, the encoding task began. Immediately following the encoding task, participants completed a distraction task for five minutes, which involved filling out questionnaires unrelated to the experiment. Participants then read instructions for the recognition task, completed practice trials, and began the recognition task. After the recognition task, the EEG cap was removed, the participant was debriefed, and the study was ended.
Behavioral data analysis
Memory performance was calculated using corrected recognition (Pr) scores, which are calculated by subtracting the false alarm rate from the hit rate (Snodgrass & Corwin, 1988). The hit/false-alarm rates were calculated as the proportion of hits/false alarms relative to the total number of possible hits/false alarms (i.e., the number of trials), separately for each participant, and separately for item and associative memory.
Paralleling the ERP analysis (EEG recording), a hit was defined as an old item/pairing correctly identified as old with high confidence (i.e., a “certain” response), and a false alarm was defined as a new/recombined item/pairing incorrectly identified as old with high confidence. To assess the manner in which memories were retrieved, we used the ROC familiarity parameter d′ and the recollection parameter Ro. ROC curves were obtained by comparing the hit rate to the false alarm rate at each confidence interval, separately for each participant and separately for item and associative memory. Thus, a total of 180 empirical ROC curves were created (90 participants, with one ROC curve for item memory and one for associative memory). Each separate empirical ROC curve was then fit to the dual-process signal detection model (Yonelinas, 1994) using the excel solver function (Dodson et al., 1998), which minimizes the sum of squared errors between the empirical and predicted values to find the best fitting parameter estimates for Ro and d′. We constrained Ro to be between 0 and 1 and d′ to be greater than or equal to 0 (Parks & Yonelinas, 2015). Pr scores, d’, and Ro were calculated separately for item memory (old vs. new items) and associative memory (old pairing vs. recombined pairing).
To test for an overall memory difference between the groups depending on the type of memory, we conducted Group (Semantic vs. Visual vs. Control) x Memory Type (item memory vs. associative memory), ANOVAs on the Pr scores, Ro, and d’. Significant interactions (α = 0.05) were followed-up with Group (2) x Memory Type (2), ANOVAs for the Semantic and Visual groups (vs. the Control group) separately, in order to test for the hypothesized memory tradeoffs in the item-focused groups (Semantic and Visual) relative to the Control group. We also examined whether the reported ease of interactive imagery during encoding differed between groups using a one-way ANOVA.
EEG recording
EEG was recorded using 32 Ag/AgCL electrodes (Fp1/2, F7/8, F3/4, Fz, FC5/6, FC1/2, T7/8, C3/4, Cz, TP9/10, CP5/6, CP1/2, P7/8, P3/4, Pz, PO9/10, O1/2, Iz.) and a NeurOne Tesla amplifier (Bittium Corporation, Finland). During recording, the EEG was referenced to electrode FCz, amplified from 0.16 to 7,000 Hz, low-pass filtered at 125 Hz, and digitized at 500 Hz.
Offline EEG analysis was done by using BrainVision Analyzer 2.0 software (Brain Products, Inc.). The EEG was first referenced to the average mastoids (TP9 and TP10) and low-pass filtered at 20 Hz. Next, the EEG was segmented from 1,000 ms pre to 7,000 ms post the onset of item 1. This segmentation captured the entire encoding window for item 1 and item 2 together to allow for the potential that ERP activity varying between groups during item 1 encoding would be sustained during associative encoding following item 2 (Kamp et al., 2016). To clean the data, ocular artifacts were corrected semi-automatically using independent component analysis with the infomax algorithm. Next, segments with >30 μV/ms steps or a 200 μV difference between the maximum and the minimum amplitude within an interval of 2,000 ms were automatically marked for exclusion from the ERP analysis. To create ERP averages for the item SME, separate ERP averages were calculated for “item hit” trials and “item miss” trials. Item hits were defined as those for which item 1 was subsequently correctly identified as old with high confidence (i.e., a “certain” response), and item misses were defined as those for which item 1 was subsequently either incorrectly identified as new or was identified as old but with low confidence (i.e., a “somewhat certain” or “uncertain” response). This approach better equates the number of trials in the hit and miss categories and also limits the number of “lucky guesses” included in the hit categories (Kamp et al., 2022; Kamp & Zimmer, 2015). To create ERP averages for the associative SME, separate ERP averages were calculated for “associative hit” trials and “associative miss” trials. Associative hits were defined as those for which the pairing was subsequently correctly identified as old with high confidence or correctly identified as recombined with high confidence, whereas associative misses were defined as those for which the pairing was either incorrectly identified as old/recombined or was correctly identified as old/recombined but with low confidence. Note that there is strong trial overlap between the item hit/miss ERPs and the associative miss/hit ERPs. As a result, any main effect of “test type” (item/associative) would not be meaningful. Instead, it is the relative comparison of item hits versus item misses against associative hits versus associative misses that is of key interest.Footnote 1
A total of 79 participants (Semantic = 26; Visual = 28; Control = 25) had a sufficient number (>8) of artifact free trials per ERP average to be included in the ERP analysis. For these participants, on average, there were 91 trials per person for item hits, 24 for item misses, 72 for associative hits, and 30 trials for associative misses.
ERP analysis
For the ERP analysis (Forester, Kroneisen, et al., 2020b; Kamp et al., 2022 for comparison), we first specified two spatial clusters and two time windows (per item) to test our hypotheses (Figs. 3 and 4). The first spatial cluster was a fontal cluster (average of F7 F3 Fz F4 F8) to capture the frontal slow wave SME, and the second was a centroparietal cluster (average of CP1, CP2, P3, Pz, P4) to capture the P300 SME. To quantify the frontal slow wave SME we calculated the mean voltage at the frontal cluster between 1,000 and 2,000 ms after item onset (i.e., 1,000-2,000 ms for item 1 and 5,000-6,000 ms for item 2) for each ERP condition separately. To quantify the P300 SME, we calculated the mean voltage at the centroparietal cluster between 400 and 800 ms after item onset (i.e., 400-800 ms for item 1 and 4,400-4,800 ms for item 2) for each ERP condition separately.
Next, we conducted Group (Semantic vs. Visual vs. Control) x Subsequent Memory (hits vs. misses) x Memory Type (item memory vs. associative memory) ANOVAs for the frontal slow wave and the P300, for the first and the second item separately. Whenever the ANOVA assumption of sphericity was violated, we used a Greenhouse-Geisser correction. Significant (α = 0.05) effects were followed up with lower-level ANOVAs followed by Bonferroni-Holm corrected t-tests. Significant effects of Memory Type are only reported when they include an interaction with SME, because the Memory Type factor is only meaningful if it indicates that an SME differs between item and associative memory.
Results
Behavioral data
For all means and standard deviations of the behavioral results, see Table 1.
Imageability ratings
There was no significant difference in the interactive imageability ratings during encoding between groups, F(2, 87) = 0.63, p = 0.534, η2p = 0.01.
Memory performance
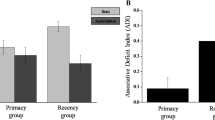
For Pr scores (Fig. 2), there was a significant main effect of Memory type on Pr scores, F(1, 87) = 8.60, p = 0.004, η2p = 0.09, qualified by a significant Group x Memory Type interaction, F(2, 87) = 5.81, p = 0.004, η2p = 0.12. To follow-up this interaction, we tested for the hypothesized memory tradeoffs for each experimental group (Semantic and Visual) relative to the Control group separately. We found that for both the Semantic [interaction: F(1, 58) = 10.81, p = 0.002, η2p = 0.16] and Visual [interaction: F(1, 58) = 5.93, p = 0.018, η2p = 0.09] groups, item memory tended to be increased while associative memory tended to be reduced relative to the Control group.
For the behavioral recollection estimate Ro (Fig. 2), there was a significant main effect of Memory type, F(1, 87) = 4.54, p = 0.036, η2p = 0.05, qualified by a significant Group x Memory Type interaction, F(2, 87) = 6.48, p = 0.002, η2p = 0.13. Matching the follow-up analysis approach used for the Pr scores, we found that for the Semantic group, F(1, 58) = 11.37, p = 0.001, η2p = 0.16, item Ro tended to be increased while associative Ro tended to be reduced relative to the Control group. The interaction was not significant for the Visual group, F(1, 58) = 0.02, p = 0.45, η2p = 0.01.
For the behavioral familiarity estimate d’, there was a significant main effect of Memory type, F(1, 87) = 4.87, p = 0.030, η2p = 0.05, reflecting that item familiarity was lower than associative familiarity overall. There was neither an interaction nor a main effect of group (both p-values > 0.225).
ERP results
The ERP waveforms and scalp distributions are presented in Figs. 3, 4 and 5. Mean ERP amplitudes are presented in Fig. 6.
Encoding frontal slow wave SMEs across the entire encoding window at the frontal electrode cluster. Time 0 ms represents the onset of the first item and time 4,000 ms represents the onset of the second item (compare the cookie and rocket ship items to Figure 1)
Encoding P300 SMEs across the entire encoding window at the centroparietal electrode cluster. Time 0 ms represents the onset of the first item and time 4,000 ms represents the onset of the second item (compare the cookie and rocket ship items to Figure 1)
(Pre-associative) item 1 frontal slow wave
For the item 1 frontal slow wave, a significant main effect of Subsequent Memory, F(1, 76) = 6.14, p = 0.015, η2p = 0.08, and Subsequent Memory x Memory type interaction, F(1, 76) =11.53, p = 0.001, η2p = 0.13, were qualified by a significant Group x Subsequent Memory x Memory Type interaction, F(1, 76) = 3.47, p = 0.036, η2p = 0.08. To follow-up this interaction, we conducted Subsequent Memory x Memory Type ANOVAs for each group. For the Semantic group, a significant main effect of Subsequent Memory, F(1, 25) = 5.68, p = 0.025, η2p = 0.19, in the absence of a significant interaction, F(1, 25) = 0.97, p = 0.759, η2p < 0.01, reflected that a more positive item 1 frontal slow wave was related to better item and associative memory. For the Visual group, a significant Subsequent Memory x Memory Type interaction, F(1, 27) = 15.84, p < 0.001, η2p = 0.37, reflected that an item 1 frontal slow wave SME was present for item memory, t(27) = 3.51, p = 0.002, d = 0.66, while a reverse frontal slow wave SME, t(27) = 2.36, p = 0.032, d = 0.43, was present for associative memory (i.e., a more positive frontal slow wave to item 1 was related to better item memory and worse associative memory for the Visual group). No significant item 1 SME, F(1, 24) = 2.62, p = 0.118, η2p = 0.10, nor a Subsequent Memory x Memory Type interaction, F(1, 24) = 3.47, p = 0.594, η2p = 0.01, was found for the Control group, suggesting that the frontal slow wave to item 1 was not reliably associated with item or associative memory in this group.
(Pre-associative) item 1 P300
For the item 1 P300, there was a main effect of Subsequent Memory, F(1, 76) = 10.10, p = 0.002, η2p = 0.12, which was qualified by a Subsequent Memory x Memory Type interaction, F(1, 76) = 4.48, p = 0.031, η2p = 0.06. The Group x Subsequent Memory x Memory Type interaction was not significant, F(2, 76) = 0.77, p = 0.467, η2p = 0.02. Follow-up tests for the Subsequent Memory x Memory Type interaction indicated that a more positive item 1 P300 was related to better item memory, t(78) = 3.75, p < 0.001, d = 0.42, but not better associative memory, t(78) = 0.81, p = 0.419, d = 0.09.
Item 2 frontal slow wave
For the item 2 frontal slow wave, we found a main effect of group, F(1, 25) = 5.00, p = 0.009, η2p = 0.12, reflecting that the Control group had a more positive frontal slow wave at the time of item 2 and inter-item associative encoding than the Visual, t(51) = 2.77, p = .008, d = 0.76, and Semantic, t(49) = 2.75, p = 0.008, d = 0.77, groups, while there was no difference between the Visual and Sematic groups, t(52) = 0.02, p = 0.985, d = 0.01.
Item 2 P300
For the item 2 P300, there was a significant main effect of Group, F(2, 76) = 7.15, p = 0.001, η2p = 0.16, which reflected that the P300 to item 2 was greater in the Control group compared to the Visual group, t(51) = 2.82, p = 0.007, d = 0.78, and showed a nonsignificant pattern for being greater compared to the Semantic group, t(49) = 1.82, p = 0.075, d = 0.51, while there was no difference between the Visual and Semantic Groups, t(52) = 0.62, p = 0.537, d = 0.17. Although there was a descriptive pattern for an item 2 P300 SME, it was not statistically significant, F(1, 76) = 2.85, p = 0.096, η2p = 0.04.
Discussion
The present study is the first to examine how isolated item encoding processes affect item and associative memory. Behaviorally, visual or semantic pre-associative item elaboration tended to enhance item memory at the expense of associative memory. A reduced slow wave and P300 during associative encoding suggest that the pre-associative item elaboration depleted or shifted resources away from associative encoding, contributing to the relative associative deficit. Importantly, the frontal slow wave during pre-associative item elaboration correlated with subsequent associative memory, with qualitative differences in this correlation depending on whether the items were elaborated semantically or visually. Our findings demonstrate that the way a single item is encoded can influence memory for of an association that includes the item.
Elaborative pre-associative item encoding instructions are linked to a relative impairment in associative memory
Extending previous studies that demonstrated a tradeoff between item and associative memory when both are encoded concurrently (Hockley & Cristi, 1996), our behavioral results revealed a similar tradeoff when an item-specific focus was adopted before associative encoding. Specifically, pre-associative item elaboration, whether semantic or visual, led to an enhancement in item memory relative to an impairment in associative memory, compared with the Control group. This effect was found despite all groups having the same time and instruction for associative memory encoding, and despite associative memory being tested only following successful item memory. The tradeoff was found for overall memory performance (Pr scores) and for estimates of recollection (Ro), but not for estimates of familiarity (d’). Because recollection refers to the retrieval of episodic detail associated with a memory (Yonelinas, 1994), these findings suggest that the performance tradeoff reflected differences in participants’ ability to consciously “remember” the items and their associations, rather than differences in the ability to automatically feel or “know” that they had been seen before (Tulving, 1985).
To examine how pre-associative item elaboration may have affected associative encoding, it is useful to examine the ERP patterns elicited by the second item. Relative to the Control group, the Semantic and Visual groups tended to have a smaller overall frontal slow wave and a smaller overall P300 following the presentation of the second item, when associative encoding became possible. This suggests that participants who utilized these intensive item-encoding strategies engaged in less associative encoding once the opportunity was available to them (frontal slow wave), and that fewer resources were allocated already during the initial encoding of the second item (P300). Together, these results support the view that pre-associative item elaboration enhances current item encoding but impairs later item and inter-item encoding, thus reducing associative memory performance.
How did this tradeoff occur, given the temporal gap that separated pre-associative item encoding from associative encoding? As outlined in the introduction, there is a set of closely related possible explanations conceptualized in terms of cognitive resources. For example, the pre-associative item focus could have diverted resources away from preparatory processes that would have aided in the ensuing associative encoding (Otten et al., 2006). Alternatively, the intensive pre-associative item focus may have depleted memory encoding resources, thus leaving these resources temporarily unavailable for the ensuing associative encoding (Popov & Reder, 2020). Finally, the item focus could have led to a strategic shift in prioritization, such that participants implicitly or explicitly deemed associative memory as less important, leading to reduced resource allocation towards associative encoding (Hockley & Cristi, 1996).
The manner of item elaboration matters for associative memory: pre-associative fontal slow wave and P300 SMEs
Based on the behavioral data and the ERPs elicited by the second item alone, it might appear that while pre-associative item elaboration does affect associative memory, the manner of this elaboration is largely irrelevant. However, a more nuanced picture emerges through investigation of the neural activity recorded during pre-associative item encoding.
First, a pre-associative P300 SME was related to better item memory, but not better associative memory for its pairing, across all groups. This indicates that early, lower-level item encoding processes were relevant to subsequent item memory, but irrelevant to associative memory, regardless of item elaboration strategies. Thus, the pre-associative ERP group differences discussed below did not reflect general group differences in motivation or similar factors (Johnson, 1986). Notably, this P300 SME result also provides novel support to a growing body of evidence for the dissociation between P300 and frontal slow wave SMEs, with the P300 SME specifically reflecting processes important to the encoding of a single (or unitized) item, and the frontal slow wave reflecting elaborative or associative processes that can influence item and associative memory (Kamp et al., 2017).
Group differences did, however, emerge within the frontal slow wave related to pre-associative item elaboration. In the Control group, where individuals presumably encoded the isolated first item relatively passively, we found that the frontal slow wave elicited during the encoding of this item was unrelated to subsequent memory. It is worth noting that this pattern deviates from our prior study, in which we did find a frontal slow wave SME in a similar design (Kamp et al., 2022). Key differences between the present study and our prior study that could have caused this dissociation are further discussed in Enhancing associative memory by changing the item focus?.
In the Semantic group, we found that the pre-associative frontal slow wave elicited by the first item was related to better subsequent memory for the item itself (item SME) and for the inter-item association that it would later become part of (associative SME). Thus, greater semantic elaboration for a given item was related to improved item and associative memory, providing support for the hypothesis that item-to-item differences in the magnitude of semantic item elaboration is related to subsequent associative memory retrieval success and hence can scaffold inter-item associations (Craik & Tulving, 1975; Madan et al., 2010; Paivio, 1963).
In the Visual group, we similarly found that the pre-associative frontal slow wave was related to better item memory (item SME). However, in stark contrast to the Semantic group, it was related to worse associative memory (reverse associative SME). Thus, greater visual item elaboration, though tied to better memory for the item, was related to worse associative memory for that item’s paring. This dissociation between the pre-associative SMEs in the frontal slow wave provides clear evidence that the manner, and not just the intensity, of pre-associative item encoding can influence subsequent associative memory. The finding is also reminiscent of results from de Chastelaine and Rugg (2015), who found that pre-item encoding fMRI SMEs in the hippocampus reversed direction when encoding was non-semantic (vs. semantic), thus similarly showing that interactions between the nature of pre-encoding and encoding processes can be associated with qualitative shifts in subsequent memory.
Putting together the behavioral and the ERP patterns, the question arises of why the behavioral associative memory deficit was similar in the Semantic and Visual groups, when pre-associative elaboration was related to better associative memory within the Semantic group and worse associative memory in the Visual group. This unexpected finding could reflect differences in associative encoding strategy between the two groups, such that the item encoding instructions may have affected the way participants applied interactive imagery. Alternatively, it could reflect more complex interactions between encoding and retrieval. For example, item recollection was particularly high in the Semantic group during memory retrieval (Fig. 2). Increased recollection of episodic item detail (which was tested first) may therefore have disrupted the ability to retrieve the more arbitrary inter-item associative relationships in some cases, such as when the items of a pair were semantically highly incongruent (Tulving & Thomson, 1973). One approach to address this issue in future research could be to manipulate item elaboration strategy orthogonally to the congruity of items.
Enhancing associative memory by changing the item focus?
Because associative memory is particularly vulnerable to factors such as aging and psychopathology, there is an interest in developing recommendations and strategies for optimizing associative memory. Our behavioral results and the ERPs to the second item of a pair suggest that perhaps the best way to support associative memory is to focus all cognitive resources on associative encoding, rather than elaborating on item features. However, the pre-associative encoding ERPs hint that appropriate pre-associative encoding strategies could potentially enhance both item and associative memory overall, rather than leading to a tradeoff. Specifically, if the detriment to associative memory reflects a cost in resource allocation during associative encoding, then mitigating this cost might negate the associative deficit (Popov & Reder, 2020). The influence of semantic item elaboration (Section The manner of item elaboration matters for associative memory: pre-associative fontal slow wave and P300 SMEs) could potentially lead to an enhancement in both item and associative memory. Future research should test this idea by manipulating, for example, 1) the temporal gap (and thus the possibility for resource competition) between item and associative encoding, 2) the manner of associative encoding, and 3) the emphasis on item vs. associative encoding. For example, 1) all items could be encoded en masse in a separate task before an associative encoding task, 2) a weaker associative encoding strategy could be used, thus rendering the cost less important, or 3) participants could be instructed to prioritize associative encoding regardless of item encoding strategy.
Additional considerations
An advantage of the present study is that associative memory was tested only when the first item was judged as old, because this avoided conflating associative memory with item 1 memory. However, this approach also has disadvantages. Because the associative memory test was dependent on item memory, it is possible that associative memory performance was influenced by item 1 memory in ways uncontrolled for in the present study. This issue is also relevant to the ROC estimation of familiarity and recollection (for discussion of a potentially related issue, see Galvin et al., 2003; Maniscalco & Lau, 2014), as well as to the ERP analysis. Thus, it will be important to replicate these findings with alternative approaches to disentangling item and associative memory, which better or differently account for their dependence.
Further, although we were able to separate (pre-associative) item 1 and inter-item associative memory (i.e., encoding and retrieval), we could not fully separate item 2 and inter-item associative memory. Thus, effects on associative memory in the present study necessarily include potential effects on item 2. Although all participants received the same time and instructions for encoding item 2, it is worth keeping this limitation (which applies to most research on associative memory) in mind.
It is worth highlighting that, unlike many previous studies (see section Encoding ERPs and subsequent memory effects), we did not find SMEs during the associative encoding time window (i.e., following item 2). Although main effects without SMEs are somewhat common (Forester, Kroneisen, et al., 2020b; Kamp, 2020; Kamp et al., 2016), we nonetheless expected to find associative SMEs like those found in our recent study (Kamp et al., 2022). The most striking difference between the two studies is the amount of time given for encoding: 3 seconds per item in the present study vs. 2 seconds in the previous study. Indeed, other recent studies of ours that found frontal slow wave main effects without SMEs included relatively long (≥3 seconds) encoding time windows as well (Forester, Kroneisen, et al., 2020b; Kamp, 2020; Kamp et al., 2016). Thus, future research should systematically investigate how encoding time affects the frontal slow wave SME during associative encoding.
Finally, it is worth noticing that the group main effect on the slow wave during associative encoding (i.e., following item 2), although clearly evident at frontal electrodes, had a more centroparietal scalp maximum (Fig. 5). The scalp distribution of memory-related slow wave activity has been shown to vary with stimulus features (Bosch et al., 2001; Khader et al., 2007), and we recently found a similarly distributed slow wave main effect that varied with motivation during elaborative item encoding (Forester, Kroneisen, et al., 2020d). It would be useful for future research to systematically examine the factors that influence the scalp distribution of slow wave activity during episodic memory encoding.
Conclusions
The way an item is encoded in isolation, immediately before being encoded into an inter-item association, can influence memory for both the item and its association. At the aggregate level, strong pre-associative item encoding may lead to a tradeoff between (enhanced) item and (impaired) associative memory. Our data support the view that this cost to associative memory occurs due to reduced cognitive resources available for (or allocated to) associative encoding, despite the item-specific and associative encoding processes being separated in time. However, ERP activity measured during pre-associative item encoding indicated that if an intensive item focus is adopted, relatively strong semantic elaboration for a given item is related to better associative memory, while relatively strong visual elaboration is related to worse associative memory, for that item’s pairing. Taken together, there appear to be two distinct and competing influences of pre-associative item encoding on associative memory: one influence impairs associative memory by depleting or diverting limited encoding resources, and the other may enhance associative memory by scaffolding inter-item associations. Thus, if the cost in resource depletion could be avoided, for example by extending the temporal gap between item and associative encoding, pre-associative semantic item elaboration might lead to enhanced item and associative memory. Future research should therefore attempt to independently vary these two influences, as a better understanding of both factors could potentially lead to novel approaches for mitigating deficits in associative memory.
More broadly, the results of this study highlight that item and associative memory are interrelated, and importantly, that the effect of this relationship may not always be easy to observe or foresee. Indeed, while we aimed to arbitrate opposing predictions from two strong lines of memory research as to how item encoding processes should affect associative memory, we instead found evidence for both predictions at different levels of analysis. Given these findings, and the relative paucity of research directly assessing the interdependence of item and associative memory processes, it will be important for future research to probe this area more deeply. Such efforts may ultimately lead to improved or novel approaches to combating memory deficits, such as those associated with aging and psychopathology, as well as to novel insights into the basic mechanisms of memory.
Open practices statement
The data for this study are available upon request. The experiment was not preregistered.
Notes
To ensure that the strong trial overlap between item hit and associative hit/miss ERPs did not unduly influence the ERP analysis, we replicated the primary statistical analyses with only three memory outcome categories—item miss vs. associative miss vs. associative hit—and tested for polynomial (i.e., linear and quadratic) effects of memory outcome and their relationship to group. All of the key findings were replicated.
References
Adcock, R. A., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B., & Gabrieli, J. D. (2006). Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron, 50(3), 507–517.
Addante, R. J., de Chastelaine, M., & Rugg, M. D. (2015). Pre-stimulus neural activity predicts successful encoding of inter-item associations. Neuroimage, 105, 21–31.
Bein, O., Livneh, N., Reggev, N., Gilead, M., Goshen-Gottstein, Y., & Maril, A. (2015). Delineating the effect of semantic congruency on episodic memory: The role of integration and relatedness. PLoS One, 10(2), e0115624.
Bosch, V., Mecklinger, A., & Friederici, A. D. (2001). Slow cortical potentials during retention of object, spatial, and verbal information. Cognitive Brain Research, 10(3), 219–237.
Bower, G. H. (1970). Imagery as a relational organizer in associative learning. Journal of Verbal Learning and Verbal Behavior, 9(5), 529–533.
Castel, A. D., & Craik, F. I. (2003). The effects of aging and divided attention on memory for item and associative information. Psychology and Aging, 18(4), 873.
Cohen, N., Pell, L., Edelson, M. G., Ben-Yakov, A., Pine, A., & Dudai, Y. (2015). Peri-encoding predictors of memory encoding and consolidation. Neuroscience & Biobehavioral Reviews, 50, 128–142.
Craik, F. I., & Tulving, E. (1975). Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General, 104(3), 268.
de Chastelaine, M., & Rugg, M. D. (2015). The effects of study task on prestimulus subsequent memory effects in the hippocampus. Hippocampus, 25(11), 1217–1223.
Dennis, N. A., Turney, I. C., Webb, C. E., & Overman, A. A. (2015). The effects of item familiarity on the neural correlates of successful associative memory encoding. Cognitive, Affective, & Behavioral Neuroscience, 15(4), 889–900.
Dodson, C. S., Prinzmetal, W., & Shimamura, A. P. (1998). Using Excel to estimate parameters from observed data: An example from source memory data. Behavior Research Methods, Instruments, & Computers, 30(3), 517–526.
Donchin, E. (1981). Surprise!… surprise? Psychophysiology, 18(5), 493–513.
Elbich, D. B., Webb, C. E., & Dennis, N. A. (2021). The influence of item familiarization on neural discriminability during associative memory encoding and retrieval. Brain and Cognition, 152, 105760.
Fabiani, M., Karis, D., & Donchin, E. (1990). Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalography and Clinical Neurophysiology, 75(1-2), 22–35.
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191.
Forester, G., Halbeisen, G., Walther, E., & Kamp, S. M. (2020). Frontal ERP slow waves during memory encoding are associated with affective attitude formation. International Journal of Psychophysiology, 158, 389–399.
Forester, G., Kroneisen, M., Erdfelder, E., & Kamp, S. M. (2020b). Survival processing modulates the neurocognitive mechanisms of episodic encoding. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 717–729.
Forester, G., Kroneisen, M., Erdfelder, E., & Kamp, S. M. (2020c). Adaptive memory: Independent effects of survival processing and reward motivation on memory. Frontiers in Human Neuroscience, 14, 588100.
Forester, G., Kroneisen, M., Erdfelder, E., & Kamp, S. M. (2020d). Adaptive memory: Independent effects of survival processing and reward motivation on memory. Frontiers in Human Neuroscience, 14, 588100.
Galvin, S. J., Podd, J. V., Drga, V., & Whitmore, J. (2003). Type 2 tasks in the theory of signal detectability: Discrimination between correct and incorrect decisions. Psychonomic Bulletin & Review, 10, 843–876.
Gold, J. J., Hopkins, R. O., & Squire, L. R. (2006). Single-item memory, associative memory, and the human hippocampus. Learning & Memory, 13(5), 644–649.
Hajcak, G., & Foti, D. (2020). Significance?... Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), e13570.
Hockley, W. E., & Cristi, C. (1996). Tests of encoding tradeoffs between item and associative information. Memory & Cognition, 24(2), 202–216.
Johnson, R. (1986). For distinguished early career contribution to psychophysiology: Award Address, 1985: A triarchic model of P300 amplitude. Psychophysiology, 23(4), 367–384.
Johnson, R. (1995). Event-related potential insights into the neurobiology of memory systems. Handbook of Neuropsychology, 10, 135–135.
Kamp, S. M. (2020). Neurocognitive mechanisms of guided item and associative encoding in young and older adults. Brain and Cognition, 145, 105626.
Kamp, S. M., & Donchin, E. (2015). ERP and pupil responses to deviance in an oddball paradigm. Psychophysiology, 52(4), 460–471.
Kamp, S. M., & Zimmer, H. D. (2015). Contributions of attention and elaboration to associative encoding in young and older adults. Neuropsychologia, 75, 252–264.
Kamp, S. M., Lehman, M., Malmberg, K. J., & Donchin, E. (2016). A buffer model account of behavioral and ERP patterns in the Von Restorff paradigm. AIMS Neuroscience, 3(2), 181–202.
Kamp, S.-M., Bader, R., & Mecklinger, A. (2017). ERP subsequent memory effects differ between inter-item and unitization encoding tasks. Frontiers in Human Neuroscience, 11, 30.
Kamp, S. M., Forester, G., Henken, M., Vittinghoff, M., & Knopf, L. (2022). On the role of item encoding mechanisms in associative memory in young and older adults: A mass univariate ERP study. Neurobiology of Learning and Memory, 189, 107588.
Karis, D., Fabiani, M., & Donchin, E. (1984). “P300” and memory: Individual differences in the von Restorff effect. Cognitive Psychology, 16(2), 177–216.
Khader, P., Ranganath, C., Seemüller, A., & Rösler, F. (2007). Working memory maintenance contributes to long-term memory formation: Evidence from slow event-related brain potentials. Cognitive, Affective, & Behavioral Neuroscience, 7(3), 212–224.
Kilb, A., & Naveh-Benjamin, M. (2011). The effects of pure pair repetition on younger and older adults' associative memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37(3), 706.
Kronesisen, M., Forester, G., & Kamp, S. M. (in press). Neurocognitive mechanisms of the survival processing effect. In M. Toglia, H. Otgaar, J. Altarriba, & W. B. Erickson (Eds.), Interdisciplinary Perspectives and Advances in Understanding Adaptive Memory. Oxford University Press.
Liu, Z. X., Grady, C., & Moscovitch, M. (2017). Effects of prior-knowledge on brain activation and connectivity during associative memory encoding. Cerebral Cortex, 27(3), 1991–2009.
Madan, C. R., Glaholt, M. G., & Caplan, J. B. (2010). The influence of item properties on association-memory. Journal of Memory and Language, 63(1), 46–63.
Madore, K. P., & Wagner, A. D. (2022). Readiness to remember: Predicting variability in episodic memory. Trends in Cognitive Sciences, 26(8), 707–723.
Maniscalco, B., & Lau, H. (2014). Signal detection theory analysis of type 1 and type 2 data: Meta-d′, response-specific meta-d′, and the unequal variance SDT model. In S. M. Fleming & C. D. Frith (Eds.), The cognitive neuroscience of metacognition (pp. 25–66). Springer-Verlag Publishing.
Murray, L. J., & Ranganath, C. (2007). The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience, 27(20), 5515–5522.
Old, S. R., & Naveh-Benjamin, M. (2008). Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging, 23(1), 104.
Otten, L. J., & Rugg, M. D. (2001). Electrophysiological correlates of memory encoding are task-dependent. Cognitive Brain Research, 12(1), 11–18.
Otten, L. J., Quayle, A. H., Akram, S., Ditewig, T. A., & Rugg, M. D. (2006). Brain activity before an event predicts later recollection. Nature Neuroscience, 9(4), 489–491.
Paivio, A. (1963). Learning of adjective-noun paired associates as a function of adjective-noun word order and noun abstractness. Canadian Journal of Psychology/Revue Canadienne de Psychologie, 17(4), 370.
Paivio, A. (1965). Abstractness, imagery, and meaningfulness in paired-associate learning. Journal of Verbal Learning and Verbal Behavior, 4(1), 32–38.
Paller, K. A., & Wagner, A. D. (2002). Observing the transformation of experience into memory. Trends in Cognitive Sciences, 6(2), 93–102.
Parks, C. M., & Yonelinas, A. P. (2015). The importance of unitization for familiarity-based learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 41(3), 881.
Popov, V., & Reder, L. M. (2020). Frequency effects on memory: A resource-limited theory. Psychological Review, 127(1), 1.
Reder, L. M., Liu, X. L., Keinath, A., & Popov, V. (2016). Building knowledge requires bricks, not sand: The critical role of familiar constituents in learning. Psychonomic Bulletin & Review, 23(1), 271–277.
Snodgrass, J. G., & Corwin, J. (1988). Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General, 117(1), 34.
Sutton, S., Braren, M., Zubin, J., & John, E. R. (1965). Evoked-potential correlates of stimulus uncertainty. Science, 150(3700), 1187–1188.
Szekely, A., Jacobsen, T., D’Amico, S., Devescovi, A., Andonova, E., Herron, D., ... & Bates, E. (2004). A new online resource for psycholinguistic studies. Journal of memory and language, 51(2), 247–250.
Tulving, E. (1985). Memory and consciousness. Canadian Psychology/Psychologie Canadienne, 26(1), 1.
Tulving, E., & Thomson, D. M. (1973). Encoding specificity and retrieval processes in episodic memory. Psychological Review, 80(5), 352.
Van Kesteren, M. T., Ruiter, D. J., Fernández, G., & Henson, R. N. (2012). How schema and novelty augment memory formation. Trends in Neurosciences, 35(4), 211–219.
Yonelinas, A. P. (1994). Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(6), 1341.
Funding
This work was supported in part by funding from the National Institute of Mental Health (T32MH08276) and from the German Research foundation (grant number: KA 4867/4-1, recipient: S. K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Forester, G., Kamp, SM. Pre-associative item encoding influences associative memory: Behavioral and ERP evidence. Cogn Affect Behav Neurosci 23, 1059–1075 (2023). https://doi.org/10.3758/s13415-023-01102-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-023-01102-7