Abstract
Previous research has documented the influence of eating together on people’s food expectations and choices. We conducted an fMRI study to investigate the influence of the label “eating together” on behavioral and brain responses to healthy or unhealthy foods. The participants (N = 28, 13 females; mean age = 21.19) viewed food photos presented with a label of “eating together” or “eating alone” and estimated the palatability, pleasantness, and desirability of each food. The label “eating together” elicited more positive ratings for both healthy and unhealthy foods than the label “eating alone,” and this effect of social context was larger for unhealthy than healthy foods. The label “eating together” also elicited greater activation in the left insula and the right posterior insula for unhealthy foods (p = 0.001 and p = 0.004, whole-brain corrected, respectively). These findings suggest that a label of “eating together” can enhance the reward values of foods, with a potentially greater enhancement for unhealthy foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As social creatures, we spend much of our lives in the company of other people and participate in many hedonic consumption activities with others, such as watching a movie in a theater or eating at a restaurant. Compared to consuming alone, we often find hedonic activities more enjoyable when engaging with companions (Ragunathan & Corfman, 2006; Ramanathan & McGill, 2007), which can also activate a sense of co-experience (Bhargave et al., 2018). When co-experiencing and co-attending with others, people are more likely to engage in elaborative processing of the stimulus (Shteynberg et al., 2014) and allocate more cognitive resources to the co-attended stimulus (Shteynberg & Apfelbaum, 2013), resulting in better memories and behavioral learning (Shteynberg, 2015). Co-experiencing with close others is especially rewarding, as evidenced by more positive ratings of the stimuli (Boothby et al., 2017) as well as greater activation in reward-related regions of the brain (Wagner et al., 2015).
One common example of consuming together in daily life is eating with others. Eating together refers to a situation in which diners simultaneously consume food in each other’s company, regardless of whether they are sharing food or not. Eating together is more desirable than eating alone across many cultures (Bloch, 1999; Pliner & Bell, 2009; Ratner & Hamilton, 2015). Eating with others can make diners happier, presumably since pleasant food might seem to taste better when people are eating with friends than when eating alone (Herman, 2015; Hetherington et al., 2006). Even eating with a stranger without any communication can amplify the food experience such that foods are perceived as more delicious and flavorful (Boothby et al., 2014). Moreover, eating together can provide diners opportunities to communicate (Julier, 2013), facilitate social bonding (Cummings & Tomiyama, 2019; Giacoman, 2016), and alleviate feelings of loneliness (Wang et al., 2021b). Collectively, such findings suggest that eating with others is intrinsically pleasant (Dunbar, 2017).
Recent studies have also demonstrated the influence of eating together on people’s food expectations and choices. Maldoy et al. (2021) showed photos of eating scenarios to participants and asked them to estimate the pleasure induced by each eating context. The participants rated eating healthy food together as eliciting marginally more pleasure than eating the same food alone, whereas no such effect was found for eating unhealthy food. In other studies, participants reported different probabilities of choosing a certain food in scenarios of eating together and eating alone when each eating scenario was described by a sentence (Wang et al., 2021a) or a combination of a sentence and a photo (Wang et al., 2021b). For example, participants reported that they were more likely to choose unhealthy foods when eating together compared to eating alone (Wang et al., 2021b). Moreover, a recent study showed that the mere anticipation of eating with others can promote the selection of larger meals for subsequent consumption (Ruddock et al., 2021). Collectively, these findings provide empirical evidence regarding the influence of eating together on food expectations and choices when exposing participants to brief depictions of eating together without placing them in real eating scenarios.
Moreover, previous research has demonstrated that a simple label (consisting of only a phrase) can convey information about the attributes of foods and influence behavioral or brain responses to those foods. For example, Grabenhorst et al. (2008) found that presenting a label about hedonic taste benefit (e.g., “rich and delicious”) could enhance the experienced pleasantness of liquid food, indexed by both higher subjective ratings and greater activation in the orbitofrontal cortex (OFC). Similarly, the presence of this type of taste-related label also increased the expected pleasantness of foods shown in images and biased the brain’s valuation system toward certain properties of foods (Grabenhorst et al., 2013). However, it remains unclear whether labels about the social context may also modulate people’s expectations of and responses to foods.
Considering the strong influence of social context on humans’ eating behaviors (Herman, 2015; Higgs & Thomas, 2016), it seems reasonable to expect that adding a simple label about the social context of eating a certain food might also influence behavioral or brain responses to that food. While we did not intend to equate the sight of an “eating together” label with the actual co-experiencing of food, we hypothesized that the presentation of an “eating together” label might be effective enough to remind the observers of the hedonic benefits provided by such a social context and to elicit experience-based expectations about the additional rewards of eating food (a reward in itself) in this social context. Therefore, we predicted an increase in the rewarding values of a food presented with an “eating together” label compared to the same food presented with an “eating alone” label. The findings of this study might explain how food labeling can be utilized as an effective marketing strategy to enhance hedonic expectations (Harris et al., 2009) and provide novel insights into how the food labels about social context might be used to nudge consumers toward healthier food-related behaviors.
We conducted a neuroimaging study to test our research hypothesis and measured the rewarding values of presented foods by analyzing neural responses during food viewing. While the participants underwent fMRI scans, they were shown images of food with “eating together” or “eating alone” labels. In order to measure the rewarding values of these foods via behavioral data, we asked the participants to provide ratings of the following attributes for each image: (1) the palatability of the food (i.e., the pleasantness of the taste of the food), which we used to assess the hedonic component of the food reward (Berridge, 1996); (2) the pleasantness of eating the food, which we used to measure the hedonic value of the food; and (3) the desire to eat the food, which we used to assess the momentary value of the food to the participants (Rogers & Hardman, 2015). Considering the different result patterns for eating healthy and unhealthy foods in Maldoy et al. (2021), we manipulated the healthiness of food by presenting images of high- and low-calorie foods (Nook & Zaki, 2015; Tang et al., 2014) and manipulated the saliency of food healthiness via the presence or absence of a label to signify its calorie level (Grabenhorst et al., 2013).
We also measured the rewarding values of the foods by analyzing neural responses during food viewing. A range of brain regions have previously been characterized as being reward -related. Neural activity in the OFC and the insula may be particularly sensitive to the modulation of eating together (vs. eating alone) for three reasons. First, these two brain regions have been characterized as cortical hedonic hotspots for food reward because they can causally amplify the hedonic impact of palatable tastes (Morales & Berridge, 2020). Even though our participants were not actually tasting any food in this study, we did ask them to estimate the palatability of food shown as images. Second, these two brain regions have both been shown to be very sensitive to food stimuli, as they can be activated by only viewing food images (Simmons et al., 2005; Zhou et al., 2019). Third, both regions have been associated with the desire to eat (Pelchat et al., 2004). This is relevant to our study because we also used ratings of desire to eat a certain food to assess the momentary value of each food labeled with an eating context (Rogers & Hardman, 2015). Despite these speculations regarding the related regions of the brain, we chose to perform unbiased whole-brain analyses of our neuroimaging data.
Materials and Methods
Participants and Recruitment
Twenty-eight healthy, right-handed Chinese young adults (mean age = 21.19 ± 2.09 years; mean BMI = 21.73 ± 3.61; 13 females and 15 males) were recruited to take part in the present study. The participants were undergraduate or graduate students at Tsinghua University in Beijing, China. All reported having normal or corrected-to-normal vision without color blindness and having no neurological or psychological illness. They were asked not to eat for at least one hour before the study so they would be mildly hungry and motivated to eat during the experiment.
The present study was approved by the Institution Review Board of Tsinghua University, and performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Each participant gave written informed consent prior to the experiment, and received 140 Chinese Yuan (equal to approximately 21.6 USD) for taking part in this study. We used G*Power 3.1 software (Faul et al., 2007, 2009) to conduct a power analysis. We used a 2 × 2 × 2 within-participant design where a sample size of 28 could detect the effects with effect size f ≥ 0.27, statistical power = 0.80, and alpha = 0.05.
Apparatus and Stimuli
Each participant took part in this study individually, and we made sure that no other people were physically present while the participant performed the experimental task. A Pentium-based computer with E-Prime 2.0 software (Psychology Software Tools, Pittsburg, PA, USA) installed was placed in a testing room outside the scanner room and used to run the experiment. Inside the scanner room, the stimuli were presented via an LCD projector onto a rear projection screen at the head end of the scanner and viewed via an angled mirror positioned attached to the head coil. A 4-key response box was used to collect the participants’ behavioral responses inside the scanner; accordingly, we used 4-point scales for the rating task.
We selected 30 photos of foods from those used by Hare et al. (2009) (N = 27) and an image database called Food-pics (N = 3) developed by Blechert et al. (2014). We only chose foods commonly seen in the local market, and each food was presented on a black background during the experiment. Similar to Nook and Zaki (2015), we used high-caloric foods (e.g., highly processed snacks or candies) to represent unhealthy food and low-caloric foods (e.g., fruits) to represent healthy food in this study.
A label concerning the context of eating (“eating together” or “eating alone”) was presented for each food image. In order to control for the possible differences among varied partners (e.g., family, friends, or colleagues), we did not specify which person to eat with during any stage of the study. Similar to Grabenhorst et al. (2013), a label about food healthiness (low-caloric or high-caloric) was also presented for each image in half of the experimental trials. It is worth noting that Grabenhorst et al. (2013) compared the influence of the presence and absence of the healthiness labels, so we also used a similar experimental design to explore whether the presence/absence of healthiness labels can influence the effect of expected eating situations on food ratings. As shown in Fig. 1, each context or healthiness label consisted of three or four Chinese characters, and all labels were shown in white text against a black background.
Sample illustrations of images of unhealthy or healthy foods labeled with “eating together” or “eating alone” with or without healthiness labels. Note that the labels of social context might be presented above or below the food image during the study, and they are shown above the food image in this figure for illustrative purposes.
Design and Procedure
We used a 2 (social context: eating together or eating alone) × 2 (food type: healthy or unhealthy food) × 2 (healthiness label: present or absent) within-participant design, resulting in four experimental conditions for each type of food. Each food image was presented once for each experimental condition, resulting in a total of 120 trials. Each label was presented above or below the food image, and the location of each label was quasi-randomly determined for each trial, with the constraint that each label was presented at each location for an equal number of trials during the whole experiment. We used a randomized event-related design, so the 120 trials were randomly divided into 6 runs of 20 trials each. Each run started with resting for 20 s, and then 20 trials were presented in a random order. Each trial lasted 20 s on average, so each run lasted seven minutes.
As shown in Fig. 2, each trial started with presenting a food image for 4 s, followed by a fixation screen for 3 s. After that, three rating screens were presented in a random order, and each screen consisted of instructions indicating the attribute being rated and four answer options. For each food image, the participants were asked to estimate how palatable the food would be, how pleasant it would be to eat the food, and their desire to eat the food, all on a four-point scale with 1 = “not at all” and 4 = “very much.” The participants were asked to complete each rating task within 3 s. Specifically, they were instructed to use the left index, left middle, right index, or right middle finger to press one of the keys on the four-key response box to choose a number from 1 to 4. Trials were separated by a randomly jittered inter-trial interval of 2 s to 6 s, and each trial lasted 18 s to 22 s. The total scanning time was approximately 50 minutes. Before the scan started, each participant also completed 12 practice trials in the resting room outside the scanner room to familiarize themselves with the task.
Imaging Data Acquisition and Analysis
The MRI data were acquired on a Philips Ingenia 3.0T CX MRI system with a 32-channel head coil. Functional images were acquired using a T2-weighted echo-planar imaging (EPI) sequence, with the number of slices = 32, repetition time = 2000 ms, echo time = 35 ms, flip angle = 90°; thickness = 5 mm, gap = 1 mm, and resolution = 96 mm × 96 mm. In order to improve the normalization of the functional data, high-resolution T1-weighted anatomical images were acquired, with the number of slices = 160, repetition time = 8.2 ms, echo time = 3.8 ms, flip angle = 8°; thickness = 1 mm, acquisition matrix = 256 × 256 × 160 voxels, field of view (FOV) = 256 mm, voxel size = 1 mm × 1 mm × 1 mm. The phase encoding was performed in the left–right direction, and three orientations of the EPI sequence (coronal, axial, and sagittal) were randomized within each run.
We used SPM12 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB 2020a software (MathWorks, Inc., USA) to preprocess the imaging data, including making corrections for slice time and for head motion using a six-parameter rigid body algorithm. Estimated translation and rotation parameters were inspected and never exceeded 2 mm or 2°, respectively. The six estimated movement parameters were added as covariates in the first-level model. All functional images were realigned to the structural images and spatially normalized to the Montreal Neurological Institute (MNI) template with a voxel size of 3 mm × 3 mm × 3 mm, and smoothed with a Gaussian kernel of 6 mm full-width at half-maximum to decrease spatial noise.
Statistical analyses were performed using the two-stage general linear model (GLM) approach implemented in SPM12. For the analysis at the single-subject level (i.e., the first stage), an event-related design matrix was applied by using SPM’s canonical hemodynamic response function with temporal and dispersion derivatives and by modeling the 8 experimental conditions (eating alone/together × unhealthy/healthy foods × healthiness labels present/absent) during the phase of image viewing. For the second stage of the analyses, we conducted 2 (social context: eating together or eating alone) × 2 (food type: healthy or unhealthy food) × 2 (healthiness label: present or absent) repeated measures analyses of variance (ANOVAs) within the whole brain. Statistical maps were thresholded at p < 0.001 (uncorrected) for a minimal cluster size of 20 voxels, and clusters were considered significant if they passed a threshold of p < 0.05 after the family-wise error (FWE) correction.
Results
Manipulation Check
In order to double-check our manipulation of food healthiness, 17 new participants were recruited from the subject pool mentioned above to rate the images without undergoing fMRI scanning. For each food image used in the fMRI study, these participants were asked to rate how healthy the food was on a four-point scale with 1 = “not at all” and 4 = “very much.” The results revealed lower healthiness ratings for the high-caloric foods than for the low-caloric ones (1.54 vs. 3.71), F(1, 16) = 310.64, p < 0.001, ηp2 = 0.95. These results suggested that our manipulation on food healthiness was valid. Similarly, we asked these participants to rate the palatability of each food on a four-point scale. The participants gave lower scores to the high-caloric foods than to the low-caloric ones (2.85 vs. 3.38), F(1, 16) = 8.33, p = 0.011, ηp2 = 0.34. This pattern was consistent with what we found in the fMRI study.
Behavioral Results
As mentioned above, our participants were asked to complete three rating tasks for each food image and to provide each rating within 3 s. Preliminary analyses revealed a high completion rate of 97.5% for the rating tasks. The mean scores of palatability, pleasantness of eating, and desire to eat are summarized in Table 1. We performed social context × food type × healthiness label repeated measures ANOVAs on these data.
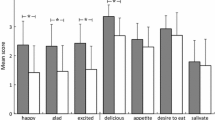
First, the results revealed a significant main effect of social context on the palatability scores [F(1, 27) = 17.51, p < 0.001, ηp2 = 0.39], the pleasantness-of-eating scores [F(1, 27) = 24.51, p < 0.001, ηp2 = 0.48], and the desire-to-eat scores [F(1, 27) = 21.36, p < 0.001, ηp2 = 0.44]. These results indicated that foods labeled with “eating together” were considered more palatable (2.91 vs. 2.77), more pleasant to eat (2.95 vs. 2.74), and more desirable (2.79 vs. 2.60) compared to the same foods labeled with “eating alone.” The main effect of food type was also significant for the palatability scores [F(1, 27) = 4.81, p = 0.037, ηp2 = 0.15], the pleasantness-of-eating scores [F(1, 27) = 4.78, p = 0.038, ηp2 = 0.15], and the desire-to-eat scores [F(1, 27) = 9.07, p = 0.006, ηp2 = 0.25]. These results indicated that the healthy foods were rated as more palatable (2.99 vs. 2.68), more pleasant to eat (3.00 vs. 2.69), and more desirable (2.93 vs. 2.47) compared to the unhealthy foods. However, these main effects were qualified by the significant social context × food type interaction terms for the palatability scores [F(1, 27) = 10.68, p = 0.003, ηp2 = 0.28], the pleasantness-of-eating scores [F(1, 27) = 7.23, p = 0.012, ηp2 = 0.21], and the desire-to-eat scores [F(1, 27) = 8.34, p = 0.008, ηp2 = 0.24].
In order to interpret the social context × food type interaction terms, we combined the data of trials with and without the healthiness labels and performed one-way repeated measures ANOVAs for each type of food separately. For the healthy foods, the main effect of social context was significant for both the pleasantness-of-eating scores [F(1, 27) = 10.00, p = 0.004, ηp2 = 0.27] and the desire-to-eat scores [F(1, 27) = 5.77, p = 0.023, ηp2 = 0.18], whereas this trend did not reach the significance level for the palatability scores [F (1, 27) = 3.19, p = 0.085, ηp2 = 0.11]. For the unhealthy foods, the main effect of social context was significant for the palatability scores [F(1, 27) = 19.11, p < 0.001, ηp2= 0.41], the pleasantness-of-eating scores [F(1, 27) = 20.91, p < 0.001, ηp2 = 0.44], and the desire-to-eat scores [F(1, 27) = 22.26, p < 0.001, ηp2 = 0.45]. Therefore, we calculated the magnitude of the social context effect by subtracting the scores of the eating-alone trials from those of the eating-together trials. As shown in Fig. 3, the results revealed a greater effect of social context on the palatability scores [F(1, 27) = 10.68, p = 0.003, ηp2 = 0.28], the pleasantness-of-eating scores [F(1, 27) = 7.23, p = 0.012, ηp2 = 0.21], and the desire-to-eat scores [F(1, 27) = 8.34, p = 0.008, ηp2 = 0.24] for the unhealthy foods than for the healthy foods.
Moreover, the social context × food type × healthiness label ANOVAs revealed a significant main effect of healthiness label on the rating of desire to eat [F(1, 27) = 6.93, p = 0.014, ηp2 = 0.20]. However, this main effect was qualified by a social context × healthiness label interaction term, which was significant for the palatability scores [F(1, 27) = 6.80, p = 0.015, ηp2 = 0.20], the pleasantness-of-eating scores [F(1, 27) = 13.02, p = 0.001, ηp2 = 0.33], and the desire-to-eat scores [F(1, 27) = 10.00, p = 0.004, ηp2 = 0.27]. None of the other main or interaction effects were significant, all Fs < 2.38, ps > 0.13. In order to interpret the significant social context × healthiness label interaction terms for all three measures, we combined the data of healthy and unhealthy foods. In the presence of healthiness labels, one-way repeated measures ANOVAs on these data revealed a significant main effect of social context on the palatability scores [F(1, 27) = 5.53, p = 0.026, ηp2 = 0.17], the pleasantness-of-eating scores [F(1, 27) = 12.67, p = 0.001, ηp2 = 0.32], and the desire-to-eat scores [F(1, 27) = 7.92, p = 0.009, ηp2 = 0.23]. In the absence of healthiness labels, one-way repeated measures ANOVAs revealed a significant main effect of social context on the palatability scores [F(1, 27) = 23.24, p < 0.001, ηp2 = 0.46], the pleasantness-of-eating scores [F(1, 27) = 34.20, p < 0.001, ηp2 = 0.56], and the desire-to-eat scores [F(1, 27) = 32.05, p < 0.001, ηp2 = 0.54]. Therefore, we calculated the magnitude of the social context effects for foods with healthiness label present and foods with healthiness labels absent separately, by subtracting the scores of the eating-alone trials from those of the eating-together trials. As can be seen in Fig. 4, one-way repeated measures ANOVAs revealed that the presentation of healthiness labels decreased the effects of social context on the palatability scores [F(1, 27) = 6.80, p = 0.015, ηp2 = 0.20], the pleasantness-of-eating scores [F(1, 27) = 13.02, p = 0.001, ηp2 = 0.33], and the desire-to-eat scores [F(1, 27) = 10.00, p = 0.004, ηp2 = 0.27].
We also performed a principal component analysis (PCA) to examine the collinearity among the three dependent variables. The results revealed that separate factors for the pleasantness of eating, palatability, and desire to eat could explain 91.7%, 6.5%, and 1.8% of the variance, respectively. These results revealed significant collinearity.
Neural Responses During Food Viewing
We conducted social context × food type × healthiness label repeated measures ANOVAs on the neuroimaging data within the whole brain. The results revealed a significant interaction term between social context and food type for the activation in the left insula, peak MNI coordinates = [−39, −12, 9], Z = 4.02, k = 46, pcorr = 0.064, and in the right posterior insula, peak MNI coordinates = [35, −27, 12], Z = 3.88, k = 107, pcorr = 0.002. None of the other main or interaction effects were significant for the left or right posterior insula. Moreover, none of the main or interaction effects were significant for the OFC or other brain regions.
In order to interpret the significant effects of the social context and food type interaction terms on the activation in the left insula and the right posterior insula, we combined the data for foods with and without the healthiness labels and performed pairwise comparisons between the foods labeled with “eating together” and “eating alone” for the healthy and unhealthy foods separately. As shown in Fig. 5, the results revealed greater activation in the left insula, peak MNI coordinates = [−33, −15, 3], Z = 3.57, k = 354, pcorr = 0.001, and in the right posterior insula, peak MNI coordinates = [51, −18, 9], Z = 3.73, k = 280, pcorr = 0.004, in response to unhealthy foods labeled with “eating together” compared to the same foods labeled with “eating alone.” As shown in Fig. 6, no such results were significant for the healthy foods after FWE corrections.
We also conducted correlational analyses to explore whether there were any significant correlations between the neuroimaging and behavioral effects in the present study. Specifically, we calculated the magnitudes of the effects of social context by subtracting the results of eating alone from those of eating together but found no significant correlations between the effects of social context on the rating scores and those on the significant insula clusters (see Table 2).
It should be noted that the presence or absence of the healthiness label was considered one of the independent variables in the present study, as the two labels were competing for the participants’ attention when a healthiness label was present. Considering that the healthiness labels were presented on some of the trials, there might be a carryover effect even if no healthiness labels were present on other trials. That is, the participants might still remember the healthiness labels even when they were absent in certain trials. In order to unconfound this carryover effect, we also combined the data of the trials with or without the healthiness labels and performed the social context × food type repeated measures ANOVAs on our neuroimaging data. The results again revealed a significant interaction term between social context and food type for the activation in the left insula, peak MNI coordinates = [−39, −24, −6], Z = 3.79, k = 235, pcorr = 0.012, and in the right insula, peak MNI coordinates = [30, −12, −6], Z = 3.59, k = 317, pcorr = 0.002. These results were in line with what we found in the social context × food type × healthiness label analyses.
Discussion
In summary, two major findings have emerged from the present study. First, the behavioral results revealed that foods labeled with “eating together” were considered more palatable, more pleasant to eat, and more desirable compared to the same foods labeled with “eating alone.” These results are in line with the positive effects of co-experiencing that previous research has documented with visual stimuli (Boothby et al., 2017; Wagner et al., 2015) or pleasant foods (Boothby et al., 2014). Our results suggest that the social context effect is very robust, as its magnitude could be decreased (presumably due to attention distraction) but not eliminated by the presentation of another label about food healthiness. These findings confirm the speculation that people expect foods to be more palatable when eating with others than when eating alone (Herman et al., 2019) and thus provide more empirical evidence regarding why eating together is more desirable than eating alone (Bloch, 1999; Pliner & Bell, 2009; Ratner & Hamilton, 2015).
Second, the behavioral and neuroimaging results of this study consistently revealed greater effects of social context for unhealthy foods than for healthy foods, even though we did not find significant correlations between the magnitudes of the social context effects in the neuroimaging and behavioral results. Specifically, we observed greater activation of the left insula and the right posterior insula in response to the unhealthy foods labeled with “eating together” compared to the same foods labeled with “eating alone” whereas no such pattern was observed for healthy foods. These findings are in line with the previously documented association between the insula and the hedonic components of food reward (Morales & Berridge, 2020), though it has been argued that not all sub-regions of the insula are involved in encoding or predicting reward (Rolls, 2004). Previous studies have consistently revealed enhanced activation in vision-associated brain areas to images of high-calorie foods compared with those of low-calorie foods, whereas they have provided less consistent evidence about enhanced activation in brain areas associated with motivational processes, such as the insula (Beaver et al., 2006; Cornier et al., 2007; Goldstone et al., 2009; Killgore et al., 2003; Passamonti et al., 2009). The insula has been suggested to play a key role in mediating expectations of the hedonic value of a food reward based on previous hedonic experience (Berridge, 2001). Therefore, the increase in the reward values of foods labeled with “eating together” may be attributed to the expectations about the additional rewards of eating the foods with other people based on previous experiences of eating together.
It should be noted that we did not intend to equate viewing an image of food labeled with “eating together” with the actual co-experiencing of food, though our findings on viewing these food images may shed light on the influence of actually eating together. The findings of this study suggest that simply adding a label of “eating together” is sufficient to remind observers of the hedonic benefits provided by such a social context (compared to eating alone), resulting in enhanced hedonic expectations as well as increased reward value for the food. Our findings are in line with the previous finding that a simple label can convey crucial information about a food and thus influence both the subjective ratings of foods and activation in reward-related regions of the brain (Grabenhorst et al., 2008, 2013).
For the foods selected for the present study, consistent findings about palatability ratings were obtained from the participants of our fMRI study and an additional experiment was conducted for the manipulation check. Similar patterns (i.e., a higher rating of palatability and greater desire to eat for low-caloric foods than for high-caloric foods) were also obtained with varied sets of food images and measure scales, such as the normative data of the Food-pics database that Blechert et al. (2014) obtained with 1,988 participants and 100-point scales or the results of (Wang et al., 2021b) obtained with a different set of eight images and seven-point scales. Similar reasons may account for the discrepancy between our study and Maldoy et al. (2021). That is, we obtained a more substantial effect of eating together for unhealthy foods than for healthy foods, whereas this effect of eating together was only marginally significant for healthy foods and not significant for unhealthy foods in Maldoy et al. (2021).
It should be noted that unhealthy foods were rated as being less palatable, less pleasant to eat, and less desirable than healthy foods in the present study. These results are inconsistent with the intuitive belief that healthiness and hedonic tastes are inversely correlated with unhealthy foods being perceived to be more palatable (Raghunathan et al., 2006). However, it should be noted that healthiness and hedonic tastes can be positively correlated, at least for some consumers (Werle et al., 2013). Alternatively, the observed difference in the palatability ratings of healthy and unhealthy foods may depend on the specific foods being rated and/or certain characteristics of the participants. Considering that the unhealthy foods were rated as being less palatable than the healthy foods in the present study, it is possible that a label of “eating together” (vs. “eating alone”) may lead to a greater increase in palatability for foods perceived to be less palatable compared to more palatable foods. This possibility needs to be tested in future research.
As for the neuroimaging results of this study, we did not observe enhanced orbitofrontal activation for foods labeled with “eating together” compared to those labeled with “eating alone.” Several possible factors may account for the absence of the orbitofrontal effect. For one, even though co-experiencing did elicit greater activation of the OFC in previous research (Schilbach et al., 2010; Wagner et al., 2015), our participants did not actually co-experience anything with anyone or have anyone else present during the experiment. Second, we compared brain responses to the same images of foods labeled with different eating contexts, but it seems more difficult to find increased orbitofrontal activation when the control stimuli are food images than when they are non-food images (Zhou et al., 2019). Similarly, we did not observe greater activation in other reward-related regions that have been shown to be sensitive to food rewards, such as the anterior cingulate cortex, amygdala, or putamen (e.g., Grabenhorst et al., 2013; Sescousse et al., 2013; van Rijn et al., 2017). Collectively, the findings of this study suggest that insula might be more sensitive to the influence of social context than other reward-related regions.
As with any study, this study has certain limitations regarding the interpretation and generalizability of the results. First, we mainly focused on the comparison between eating together and eating alone in the present study, so we did not specify whether foods would be shared or not in the context of eating together (De Backer et al., 2015; Wang et al., 2021a; Woolley & Fishbach, 2019). Second, we only tested young participants with normal weight in the present study, whereas previous research has demonstrated a difference in overweight and normal-weight participants’ brain responses to foods, especially for high-caloric foods (Murdaugh et al., 2012). Therefore, caution is called for in attempting to generalize our findings to a population with a wider range of BMI, other age groups, or non-student samples (Henrich et al., 2010). Third, our results showed significant collinearity among the three rating scores. The experimental design of the present study does not allow us to further differentiate these three types of ratings, but it will be interesting and important to do so in future research. Moreover, considering that our participants rated the unhealthy foods as being less palatable than the healthy foods in the present study, caution is called for in attempting to generalize our findings to people who would rate unhealthy foods as being more palatable than healthy foods.
In conclusion, the findings of the present study revealed different behavioral and brain responses to foods labeled with “eating together” and those labeled with “eating alone.” Specifically, our findings suggest that expectations about eating unhealthy foods with others could lead to enhanced hedonic expectations and greater activation in the insula. These findings provide new empirical evidence regarding the influence of social context on people’s food-related behaviors (Herman, 2015; Higgs & Thomas, 2016) and once again highlight the social nature of human beings (Dunn et al., 2008; Yamaguchi et al., 2016). The findings of this study also provide insight into how to integrate social factors (such as eating with others) and food pleasure to promote healthy food consumption (Mendini et al., 2019). Specifically, our results demonstrate the different influences that a label conveying the social context of eating may exert on varied types of foods and shed light on the notion that different strategies should be used for the label design of healthy and unhealthy foods, presumably as unhealthy foods may become particularly rewarding when eating with others. These findings may have implications for the practice of nudging people toward healthier food choices, such as presenting pictorial or verbal cues about a food to be eaten together with others on food packaging, especially for foods that are considered appropriate for sharing.
References
Beaver, J. D., Lawrence, A. D., Van Ditzhuijzen, J., Davis, M. H., Woods, A., & Calder, A. J. (2006). Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience, 26(19), 5160-5166. https://doi.org/10.1523/JNEUROSCI.0350-06.2006
Berridge, K. C. (1996). Food reward: Brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews, 20, 1-25. https://doi.org/10.1016/0149-7634(95)00033-B
Berridge, K. C. (2001). Reward learning: Reinforcement, incentives, and expectations. Psychology of Learning and Motivation, 40, 223-278. https://doi.org/10.1016/S0079-7421(00)80022-5
Bhargave, R. P., Montgomery, N. V., & Redden, J. P. (2018). Collective satiation: How coexperience accelerates a decline in hedonic judgments. Journal of Personality and Social Psychology, 114, 529-546. https://doi.org/10.1037/pspa0000099
Blechert, J., Meule, A., Busch, N. A., & Ohla, K. (2014). Food-pics: An image database for experimental research on eating and appetite. Frontiers in Psychology, 5: 617. https://doi.org/10.3389/fpsyg.2014.00617
Bloch, M. (1999). Commensality and poisoning. Social Research, 66(1), 33-149. https://doi.org/10.2307/40971306
Boothby, E. J., Clark, M. S., & Bargh, J. A. (2014). Shared experiences are amplified. Psychological Science, 25, 2209-2216. https://doi.org/10.1177/0956797614551162
Boothby, E. J., Smith, L. K., Clark, M. S., & Bargh, J. A. (2017). The world looks better together: How close others enhance our visual experiences. Personal Relationships, 24(3), 694-714. https://doi.org/10.1111/pere.12201
Cornier, M. A., Von Kaenel, S. S., Bessesen, D. H., & Tregellas, J. R. (2007). Effects of overfeeding on the neuronal response to visual food cues. American Journal of Clinical Nutrition, 86(4), 965-971. https://doi.org/10.1093/ajcn/86.4.965
Cummings, J. R., & Tomiyama, A. J. (2019). Food loves company: Risky eating with friends increases interpersonal closeness. Journal of Experimental Social Psychology, 81, 61-69. https://doi.org/10.1016/j.jesp.2018.07.006
De Backer, C. J. S., Fisher, M. L., Poels, K., & Ponnet, K. (2015). “Our” food versus “my” food. Investigating the relation between childhood shared food practices and adult prosocial behavior in Belgium. Appetite, 84, 54-60. https://doi.org/10.1016/j.appet.2014.09.022
Dunbar, R. I. M. (2017). Breaking bread: The functions of social eating. Adaptive Human Behavior and Physiology, 3, 198-211. https://doi.org/10.1007/s40750-017-0061-4
Dunn, E. W., Aknin, L. B., & Norton, M. I. (2008). Spending money on others promotes happiness. Science, 319(5870), 1687-1688. https://doi.org/10.1126/science.1150952
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. https://doi.org/10.3758/BF0319
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. https://doi.org/10.3738/BRM.41.4.1149
Giacoman, C. (2016). The dimensions and role of commensality: A theoretical model drawn from the significance of communal eating among adults in Santiago, Chile. Appetite, 107, 460-470. https://doi.org/10.1016/j.appet.2016.08.116
Goldstone, A. P., Prechtl de Hernandez, C. G., Beaver, J. D., Muhammed, K., Croese, C., Bell. et al. (2009). Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience, 30(8), 1625-1635. https://doi.org/10.1111/j.1460-9568.2009.06949.x
Grabenhorst, F., Rolls, E. T., & Bilderbeck, A. (2008). How cognition modulates affective responses to taste and flavor: Top-down influences on the orbitofrontal and pregenual cingulate cortices. Cerebral Cortex, 18, 1549-1559. https://doi.org/10.1093/cercor/bhm185
Grabenhorst, F., Schulte, F. P., Maderwald, S., & Brand, M. (2013). Food labels promote healthy choices by a decision bias in the amygdala. NeuroImage, 74, 152-163. https://doi.org/10.1016/j.neuroimage.2013.02.012
Hare, T. A., Camerer, C. F., & Rangel, A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646-648. https://doi.org/10.1126/science.1168450
Harris, J. L., Brownell, K. D., & Bargh, J. A. (2009). The food marketing defense model: Integrating psychological research to protect youth and inform public policy. Social Issues and Policy Review, 3, 211-271. https://doi.org/10.1111/j.1751-2409.2009.01015.x
Henrich, J., Heine, S. J., & Norenzayan, A. (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33(2-3), 61-83. https://doi.org/10.1017/S0140525X0999152X
Herman, C. P. (2015). The social facilitation of eating. A review. Appetite, 86, 61-73. https://doi.org/10.1016/j.appet.2014.09.016
Herman, C. P., Polivy, J., Pliner, P., & Vartanian, L. R. (2019). Social influences on eating. : Springer Nature Switzerland. https://doi.org/10.1007/978-3-030-28817-4
Hetherington, M. M., Anderson, A. S., Norton, G. N., & Newson, L. (2006). Situational effects on meal intake. A comparison of eating alone and eating with others. Physiology & Behavior, 88, 498-505. https://doi.org/10.1016/j.physbeh.2006.04.025
Higgs, S., & Thomas, J. (2016). Social influences on eating. Current Opinion in Behavioral Sciences, 9, 1-6. https://doi.org/10.1016/j.cobeha.2015.10.005
Julier, A. P. (2013). Eating together: Food, friendship, and inequality. : University of Illinois Press.
Killgore, W. D., Young, A. D., Femia, L. A., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. A. (2003). Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage, 19(4), 1381-1394. https://doi.org/10.1016/S1053-8119(03)00191-5
Maldoy, K., De Backer, C. J. S., & Poels, K. (2021). The pleasure of sharing: Can social context make healthy food more appealing? Psychology & Marketing, 38(2), 359-370. https://doi.org/10.1002/mar.21396
Mendini, M., Pizzetti, M., & Peter, P. C. (2019). Social food pleasure: When sharing offline, online and for society promotes pleasurable and healthy food experiences and well-being. Qualitative Market Research, 22(4), 544-556. https://doi.org/10.1108/QMR-06-2018-0067
Morales, I., & Berridge, K. C. (2020). Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiology & Behavior, 227: 113152. https://doi.org/10.1016/j.physbeh.2020.113152
Murdaugh, D. L., Cox, J. E., Cook, E. W., & Weller, R. E. (2012). fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage, 59(3), 2709-2721. https://doi.org/10.1016/j.neuroimage.2011.10.071
Nook, E. C., & Zaki, J. (2015). Social norms shift behavioral and neural responses to Foods. Journal of Cognitive Neuroscience, 27(7), 1412-1426. https://doi.org/10.1162/jocn_a_00795
Passamonti, L., Rowe, J. B., Schwarzbauer, C., Ewbank, M. P., Von Dem Hagen, E., & Calder, A. J. (2009). Personality predicts the brain's response to viewing appetizing foods: The neural basis of a risk factor for overeating. Journal of Neuroscience, 29(1), 43-51. https://doi.org/10.1523/JNEUROSCI.4966-08.2009
Pelchat, M. L., Johnson, A., Chan, R., Valdez, J., & Ragland, J. D. (2004). Images of desire: Food craving activation during fMRI. NeuroImage, 23, 1486-1493. https://doi.org/10.1016/j.neuroimage.2004.08.023
Pliner, P., & Bell, R. (2009). A table for one: The pain and pleasure of eating alone. In Meals in science and practice: Interdisciplinary research and business applications (pp. 169-189). Woodhead Publishing.
Ragunathan, R., & Corfman K. (2006). Is happiness shared doubled and sadness shared halved? Social influence on enjoyment of hedonic experiences. Journal of Marketing Research, 43, 386-394. https://doi.org/10.1509/jmkr.43.3.386
Raghunathan, R., Naylor, R. W., & Hoyer, W. D. (2006). The unhealthy=tasty intuition and its effects on taste inferences, enjoyment, and choice of food products. Journal of Marketing, 70(4), 170-184. https://doi.org/10.1509/jmkg.70.4.170
Ramanathan, S., & McGill, A. L. (2007). Consuming with others: Social influences on moment-to-moment and retrospective evaluations of an experience. Journal of Consumer Research, 34, 506-524. https://doi.org/10.1086/520074
Ratner, R. K., & Hamilton, R. W. (2015). Inhibited from bowling alone. Journal of Consumer Research, 42, 266-283. https://doi.org/10.1093/jcr/ucv012
Rogers, P. J., & Hardman, C. A. (2015). Food reward. What it is and how to measure it. Appetite, 90, 1-15. https://doi.org/10.1016/j.appet.2015.02.032
Rolls, E. T. (2004). Smell, taste, texture, and temperature multimodal representations in the brain, and their relevance to the control of appetite. Nutrition Reviews, 62(11), S193-S204. https://doi.org/10.1111/j.1753-4887.2004.tb00099.x
Ruddock, H. K., Long, E. V., Brunstrom, J. M., Vartanian, L. R., & Higgs, S. (2021). People serve themselves larger portions before a social meal. Scientific reports, 11(1), 1-8. https://doi.org/10.1038/s41598-021-90559-y
Schilbach, L., Wilms, M., Eickhoff, S. B., Romanzetti, S., Tepest, R., Bente, G. et al. (2010). Minds made for sharing: Initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience, 22(12), 2702-2715. https://doi.org/10.1162/jocn.2009.21401
Sescousse, G., Caldú, X., Segura, B., & Dreher, J.-C. (2013). Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37, 681-696. https://doi.org/10.1016/j.neubiorev.2013.02.002
Shteynberg, G. (2015). Shared attention. Perspectives on Psychological Science, 10, 579-590. https://doi.org/10.1177/1745691615589104
Shteynberg, G., & Apfelbaum, E. P. (2013). The power of shared experience: Simultaneous observation with similar others facilitates social learning. Social Psychological & Personality Science, 4, 738-744. https://doi.org/10.1177/1948550613479807
Shteynberg, G., Hirsh, J. B., Galinsky, A. D., & Knight, A. P. (2014). Shared attention increases mood infusion. Journal of Experimental Psychology: General, 143, 123-130. https://doi.org/10.1037/a0031549
Simmons, W. K., Martin, A., & Barsalou, L. W. (2005). Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex, 15(10), 1602-1608. https://doi.org/10.1093/cercor/bhi038
Tang, D. W., Fellows, L. K., & Dagher, A. (2014). Behavioral and neural valuation of foods is driven by implicit knowledge of caloric content. Psychological Science, 25(12), 2168-2176. https://doi.org/10.1177/0956797614552081
van Rijn, I., Wegman, J., Aarts, E., de Graaf, C., & Smeets, P. A. M. (2017). Health interest modulates brain reward responses to a perceived low-caloric beverage in females. Health Psychology, 36(1), 65-72. https://doi.org/10.1037/hea0000411
Wagner, U., Galli L., Schott, B., Wold, A., Manstead, A., Scherer, K. R., & Walter, H. (2015). Beautiful friendship: Social sharing of emotions improves subjective feelings and activates the neural reward circuitry. Social Cognitive and Affective Neuroscience, 10(6), 801-808. https://doi.org/10.1093/scan/nsu121
Wang, C., Huang, J., & Wan, X. (2021a). A cross-cultural study of beliefs about the influence of food sharing on interpersonal relationships and food choices. Appetite, 161: 105129. https://doi.org/10.1016/j.appet.2021.105129
Wang, C., Peng, Y., Qiu, L., & Wan, X. (2021b). Cloud-based commensality: Enjoy the company of co-diners without social facilitation of eating. Frontiers in Psychology, 12:758966. https://doi.org/10.3389/fpsyg.2021.758966
Werle, C. O., Trendel, O., & Ardito, G. (2013). Unhealthy food is not tastier for everybody: The “healthy = tasty” French intuition. Food Quality and Preference, 28(1), 116-121. https://doi.org/10.1016/j.foodqual.2012.07.007
Woolley, K., & Fishbach, A. (2019). Shared plates, shared minds: Consuming from a shared plate promotes cooperation. Psychological Science, 30(4), 541-552. https://doi.org/10.1177/0956797619830633
Yamaguchi, M., Masuchi, A., Nakanishi, D., Suga, S., Konishi, N., Yu, Y., & Ohtsubo, Y. (2016). Experiential purchases and prosocial spending promote happiness by enhancing social relationships. Journal of Positive Psychology, 11(5), 480-488. https://doi.org/10.1080/17439760.2015.1117128
Zhou, Y., Gao, X., Small, D. M., & Chen, H. (2019). Extreme spicy food cravers displayed increased brain activity in response to pictures of foods containing chili peppers: An fMRI study. Appetite, 142: 104379. https://doi.org/10.1016/j.appet.2019.104379
Acknowledgments
The authors would like to thank Yuxuan Qi and Yubin Peng for their help in some of the data collection.
Funding
This work was supported by Tsinghua University Spring Breeze Fund awarded to Xiaoang Wan and General Project of Philosophy and Social Science Research in Universities of Jiangsu Province (Grant No. 2021SJA1345) awarded to Jianping Huang.
Author information
Authors and Affiliations
Contributions
Each of the listing co-authors made the following contributions to the paper: XW and JH co-developed the idea for the study and collaboratively designed the study. CW and JH collected the data and conducted the data analysis. JH, CW, and XW conducted the interpretation of the data. XW and JH drafted the manuscript. CW provided critical revisions. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
All the authors declare that there are no conflicts of interest.
Ethics Approval
This research was approved by the Institution Review Board of Tsinghua University, and performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Consent to participate
All the participants who took part in this study have signed informed consent.
Consent for publication
Not applicable.
Additional information
Open practices statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study was not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, J., Wang, C. & Wan, X. Influence of eating together on brain activation and hedonic evaluation in response to foods. Cogn Affect Behav Neurosci 22, 1145–1156 (2022). https://doi.org/10.3758/s13415-021-00982-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00982-x