Abstract
Threat sensitivity is thought to be a precursor for anxiety. Yet it remains unknown whether individuals have consistently high neural activation to different threatening situations. The current study (N = 161, Mage = 11.26, SD = 1.79) used three ERPs from different threat-related events: 1) the P3 to receiving negative feedback; 2) the ERN to making mistakes; 3) the N170 to viewing angry faces. Participants also completed self-report measures of threat sensitivity, impulsivity, and demographics. In a follow-up analysis, we also investigated whether the results replicate when using the difference score for each ERP. Youth with higher self-reported sensitivity to threats and lower self-reported impulsivity had consistently higher neural activation to threatening situations. Males also had consistently higher neural activation to threats compared with females. When using the difference score, we found that youth with higher self-reported threat sensitivity had consistently higher neural activation to threats than nonthreats. Although it is common for youth to have high neural activation during at least one threatening situation (e.g., making mistakes), only ~12% of youth have consistently high neural activation across a variety of different threats. Thus, detecting youth who are sensitive to a variety of different threats may be an important avenue to investigate to identify youth most at risk for the development of anxiety.
Similar content being viewed by others
Several theories of adolescent development posit that adolescence may be a time of heightened sensitivity to emotionally salient events (Casey, 2015; Somerville et al., 2010; Steinberg et al., 2008). Indeed, adolescents tend to report greater sensitivity to threat—one type of emotionally salient event—compared with children (O’Brien & Bierman, 1988; Vervoort et al., 2010). In contrast, some work suggests that adolescents may have lower threat sensitivity compared with children (Humphreys et al., 2016; McCormick & Telzer, 2017). See Ernst et al.’s (2006) Triadic Model, which posits that adolescents have a strong reward system but a weak harm-avoidant system. Thus, the relationship between threat sensitivity and development remains unclear. Of concern, heightened sensitivity to threats has been found to be associated with anxiety (Balle et al., 2013; Bar-Haim et al., 2007; Johnson et al., 2003; Katz et al., 2020; Pérez-Edgar et al., 2010, 2011; Vervoort et al., 2010); therefore, it is critical to advance our understanding of threat sensitivity among youth.
There are a variety of different situations, however, that youth may find threatening, such as receiving negative feedback, making mistakes, and seeing angry faces. In survey research, these threatening situations are generally combined into one overall measure of threat sensitivity [e.g., Behavioral Inhibition Scale (BIS; Carver & White, 1994); Sensitivity to Punishment Scale (Torrubia et al., 2001)]. For example, the BIS includes questions assessing responsiveness to negative feedback (e.g., “Criticism or scolding hurts me quite a bit”), making mistakes (e.g., “I worry about making mistakes”), and worrying about whether someone is angry at you (“I feel pretty worried or upset when I think or know somebody is angry at me”). Thus, in survey research, the assumption is that people who are high on sensitivity to threat in one situation also tend to be high in other situations. Within the neuroscience literature, however, different threatening situations are treated as distinct events, each being investigated in isolation from each other. For example, a task where someone receives negative feedback about their performance (e.g., a gambling task) is not compared to a task where someone makes mistakes (e.g., during a go/no-go task); yet both of these situations are captured within the same self-report survey. Research has yet to investigate (1) individual differences in the consistency of ERP activation across different threat-related events (e.g., Do some participants have consistently high neural activation across different threat-related events, while others show heightened neural activation to only one or two events?) and (2) what demographic and self-report factors are associated with individual differences in neural activation across different tasks (e.g., do adolescents have more consistently high neural activation to threats than children? Do individuals who self-report greater threat sensitivity have consistently higher activation across these tasks?).
ERPs to threatening situations
One way to investigate how an individual reacts to different types of threats is to consider their neural activation directly after a threatening event occurs. Event-related potentials (ERPs: an averaged EEG response that is time-locked to an event; Luck, 2005) can provide a sensitive measure of neural activation directly after a threatening event happens (e.g., after an individual receives negative feedback). We have elected to investigate three different types of threatening events that are consistent with self-report measures of threat sensitivity: receiving negative feedback, making mistakes, and viewing angry faces. Below, we discuss three ERPs that are elicited in response to these types of events and have previously been associated with self-reported threat sensitivity.
Negative feedback (P3)
The P3 is an ERP component that is associated with paying attention to feedback (Huang et al., 2015; Luck, 2005). Previous research has found that individuals who have greater sensitivity to threat tend to have larger P3 amplitudes to negative feedback than those with lower sensitivity to threat (De Pascalis et al., 2004; Heffer & Willoughby, 2020; Miltner et al., 2005). Furthermore, Reeb-Sutherland et al. (2009) found a trend whereby high sensitivity to negative feedback and larger P3 amplitudes were associated with greater anxiety.
Making mistakes (ERN)
The error-related negativity (ERN) is an ERP that is associated with performance monitoring, specifically when making mistakes during an inhibitory control task. Indeed, this ERP corresponds to the motivational significance of errors, whereby a larger ERN is associated with greater motivation to avoid errors (Hajcak & Foti, 2008; Meyer, 2017). Individuals with greater threat sensitivity or anxiety tend to have larger ERNs when making errors than those with lower threat sensitivity or anxiety (Boksem et al., 2008; Chong & Meyer, 2019; Hajcak et al., 2003; Ladouceur et al., 2006; Meyer, 2017; Meyer & Hajcak, 2019; Weinberg et al., 2010).
Viewing angry faces (N170)
N170 is an ERP that is elicited to faces (e.g., angry faces). Previous research has found that the N170 is larger in response to angry faces compared to other emotional expressions (e.g., neutral or happy Denefrio et al., 2019; Hinojosa et al., 2015; Jetha et al., 2013; Kolassa et al., 2009, Kolassa et al., 2007; Rossignol et al., 2005). Furthermore, individuals with greater threat sensitivity (or anxiety) tend to have a larger N170 to angry faces than those with less sensitivity to threat or anxiety (Bechor et al., 2019; Kolassa & Miltner, 2006; O’Toole et al., 2013; Wieser et al., 2010).
Age-related differences in ERPs
Of note, there has been some work identifying age-related differences in these ERPs across development (Downes et al., 2017). Specifically, there is evidence that the ERN (Davies et al., 2004; DuPuis et al., 2015; Kim et al., 2005; Santesso et al., 2006; Wiersema et al., 2007) and the P3 (Heffer & Willoughby, 2020; van Dinteren et al., 2014) tend to be larger among older participants. Other studies, however, have not found this pattern of age-related changes among the ERN or the P3 (Eppinger et al., 2009; Grose-Fifer et al., 2014). Age-related changes in the N170 are less consistent. Some studies have found that this ERP also gets larger with age (Hileman et al., 2011), whereas others have shown that the N170 fluctuates across development, showing more positive values around late childhood and early adolescence followed by greater negativity into adulthood (Batty & Taylor, 2006; Kuefner et al., 2010; Taylor et al., 2004). Overall, this research highlights that age may be an important factor to consider when investigating the ERN, P3, and N170. This research, however, has rarely taken into consideration individual differences that may also affect ERP amplitudes. Indeed, youth who are in the same age group have a larger N170s, ERNs or P3 when they have greater anxiety-related symptoms than when they do not have anxiety-related symptoms (Chong & Meyer, 2019; Heffer & Willoughby, 2020; O’Toole et al., 2013). Thus, it is unlikely that these changes in amplitude across development are simply the result of age.
To date, these ERPs have been investigated in isolation and no study has investigated whether individuals have neural consistency across these ERPs. In other words, it is not clear whether the same individuals have a high neural sensitivity across these different threat-related events (i.e., are individuals consistently sensitive to different threats?). Based on self-report measures of sensitivity to threat—that incorporate these different threatening situations all into one measure—we might expect that individuals will be consistently sensitive (or not) across these different situations. From a neuroscience perspective, however, these threat-related events are examined in separate lines of research and each of these ERPs are thought to have different neural generators. For example, studies that have combined fMRI and EEG have suggested that the ERN is generated in the anterior cingulate cortex (Debener et al., 2005; Mathalon et al., 2003)—an important region involved in goal-directed behavior (Holroyd & Yeung, 2012). The N170, however, is thought to be activated in face processing regions (e.g., superior temporal sulcus or the fusiform gyrus; Sadeh et al., 2010), while the P3 is a neural indicator associated with attention (Huang et al., 2015; Luck, 2005). Thus, within the neuroscience field, these different threatening events are thought to be distinct and related to different neural processes. No study has investigated individual differences in consistency of neural activation across these different tasks. Importantly, cumulative neural-level sensitivity across these different threats (e.g., across a number of situations) may be important to consider when targeting individuals at risk factors for anxiety, given that these individuals would have a high reactivity to threat across multiple situations (and multiple neural generators).
At the same time, there may be some children and adolescents who are not concerned with these threatening situations (e.g., they may be consistently low on these ERPs). Indeed, some individuals may be less bothered by these types of threats and therefore may pay less attention to negative feedback, errors, or angry faces. For instance, impulsive individuals tend to be less concerned with threatening situations and instead engage in nonreflective, stimulus-driven responses (Nigg, 2017). Previous research has found that impulsive individuals tend to have smaller P3 amplitudes (Justus et al., 2001; Ruchsow et al., 2008) and smaller ERN amplitudes (Checa et al., 2014; Pailing et al., 2002; Ruchsow et al., 2005; Stahl & Gibbons, 2007; Taylor et al., 2018) compared with individuals who are less impulsive. Less is known about whether impulsive individuals have smaller N170 activation to angry faces; thus, this latter analysis is more exploratory.
Current study
The current study seeks to assess whether consistently high neural activation to threats across different tasks is associated with both demographic and self-report factors (sensitivity to threat, impulsivity, age, pubertal status, sex, and parental education). While this analysis is exploratory, we expect that individuals who self-report higher sensitivity to threat and lower impulsivity will have consistently higher neural activation to threats. We also predict that adolescents (those with more advanced pubertal development and older age) will be more likely to have consistently higher neural activation to threats.
In a follow-up analysis, we further investigate whether the results will replicate when using a difference score for each of these threatening events (i.e., P3 loss - P3 wins; N170 angry - N170 neutral; ERN - CRN). A difference score specifically examines whether individuals have higher neural activation to threats than to non-threatening events. One difference between this analysis and the previous analysis is that difference scores offer a way to investigate whether individuals have neural activation that is specific to threats. In other words, difference scores provide a method for checking if individuals have greater activation to threats than non-threats. At the same time, however, only investigating difference scores can sometimes make interpretation of the results unclear. For example, an individual who has high neural activation to receiving negative feedback and high activation to receiving positive feedback would have a low difference score, but their score could be identical to a person who has low neural activation to negative feedback and low neural activation to positive feedback. Given the different strengths and weaknesses of these analyses, we include both an analysis using only the threat-related ERPs and an analysis using the difference score.
Method
Participants
Participants (N = 228, Mage = 10.57, SD = 1.77; age range = 8-14 years, 49.36% female) were drawn from several elementary and high schools in southern Ontario, Canada, and were part of a larger study examining the associations between wellbeing and youth health-risk behaviors. Parent report indicated that 82.96% of the children and adolescents were white, 1.89% were black, 1.42% were Asian, 2.36% were Hispanic, 0.47% indigenous, and 9.43% were mixed (0.47% of parents indicated that they preferred not to answer the question).
Procedure
Students were invited to participate in the study through visits to schools. Surveys were completed in classrooms during school hours, and all participants received gifts (e.g., backpacks) as compensation. Participants also completed a Mobile Lab component in which EEG data were recorded. Parents were asked to identify if their child had any illnesses or disabilities (either physical or mental). One participant was excluded because of a diagnosis of autism. Eleven people were excluded because of equipment issues (e.g., the event markers did not show up) on at least one of the tasks. Fifteen people were excluded, because EEG data were not usable (e.g., contained a larger number of muscle/movement artifacts) on at least one of the tasks. Two participants did not complete one of the tasks, and five participants did not follow the instructions (e.g., they were off task). We also had 33 participants who had less than 6 trials on the ERN, which can be cause for concern (Olvet & Hajcak, 2009). Thus, we removed these participants from our analyses. The final sample included 161 participants. Of note, the final sample was fairly equally distributed among children aged 8-11 years (N = 91) and adolescents aged 12-14 years (N = 70). The University Ethics Board approved this study. Participants provided informed assent, and their parents provided informed consent.
Missing Data Analysis
Missing data occurred because some participants did not finish the questionnaire (average missing data = 5.09%) and because some participants were absent during the time of the survey. The percentage of students who completed the survey was 93.43%. Missing data were primarily due to absenteeism, but also occasionally due to time conflicts, RA mistakes (e.g., not inviting a child to complete the survey), or students moving to another school district with no contact information. Missing data were imputed using the expectation-maximization algorithm (EM). EM retains cases that are missing survey waves and thus avoids the biased parameter estimates that can occur with pairwise or listwise deletion (Schafer & Graham, 2002).
Measures
Demographics
Pubertal status, age, sex, and parental education were collected. Parental education was measured with one item per parent on the following scale: 1 (did not finish high school); 2 (high school diploma); 3 (some university/college); 4 (associate degree/diploma); 5 (undergraduate degree); 6 (graduate degree). The average level of parental education for this sample was a 4, “completed an associate degree and/or technical diploma.” Pubertal status was assessed using the Puberty Development Scale (PDS; Petersen et al., 1988). The PDS is a self-report measure that assesses body hair, facial hair, and voice development in boys, and body hair, menarche, and breast development in girls. All items were rated on a 4-point scale from 1 (not yet started changing) to 4 (change seems complete). The PDS scale exhibits good reliability and validity (Carskadon & Acebo, 1993; Petersen et al., 1988).
Sensitivity to Threat
Participants reported the extent to which they agreed with three items specifically examining sensitivity to threat from the Behavioral Inhibition Scale (Carver & White, 1994; “Criticism hurts me quite a bit”; “I feel worried when I think I have done poorly at something”; “I feel pretty worried or upset when I think or know somebody is angry at me”) on a scale ranging from 1 (strongly disagree) to 4 (strongly agree). Higher scores indicate higher levels of threat sensitivity. Cronbach’s alpha was 0.73.
Impulsivity
Impulsivity was measured using 4 items (“I do not consider the consequences before I act”; “I say things without thinking”; “I often act on the spur of the moment”; “I do things without thinking”; Baars et al., 2015; Barratt, 1959; Patton et al., 1995; Van der Elst et al., 2012). Items were assessed on a 4-point scale from 1 (almost never) to 4 (almost always). Higher scores indicate higher impulsivity. Cronbach’s alpha for this scale was 0.79.
Go/No-go task
Participants completed the go/no-go task (DuPuis et al., 2015) while EEG was recorded. Participants were instructed to continuously push a button every time a stimulus appeared (a Go trial) unless the newly presented stimulus matched the previously presented stimulus (i.e., the same stimulus appeared twice in a row), in which case the participant needed to refrain from pushing the button on that trial (a No-go Trial). We were particularly interested in the ERN, an ERP elicited when participants make mistakes during this task. Stimuli were presented 1,000-ms apart, and there were a total of 225 trials. On average, participants committed 17 errors (SD = 8.25) on no-go trials. The average reaction time to a no-go trial was 362 ms (SD = 47.89 ms). To create the difference score, we also extracted the correct-response negativity (CRN)—an ERP elicited when participants correctly push a button during a go trial.
Balloon Analogue Risk Task
The Balloon Analogue Risk Task (BART) is a behavioral task that has been used to measure risky decision-making (Lejuez et al., 2002). We used a modified version of the BART to use this task for an ERP study (Heffer & Willoughby, 2020). Participants were instructed to inflate a series of balloons to earn points. Participants indicated the number of pumps they wanted to inflate the balloon at the beginning of the trial (Euser et al., 2013; Pleskac et al., 2008; Yau et al., 2015). Participants then observed the balloon as it either safely reached the inflation number they picked (i.e., they won the points for that trial), or the balloon burst before reaching that point (i.e., they lost the points for that trial). Given that this task provides feedback associated with losing (i.e., when the balloon pops and points are lost), it facilitates the examination of sensitivity to negative and positive feedback using ERPs (Chandrakumar et al., 2018; Fein & Chang, 2008; Gu et al., 2018; Takács et al., 2015).
The task consisted of 90 trials with a maximum breaking point of 20 pumps. The probability of the balloon popping increased as the number of pumps chosen increased (e.g., choosing to pump the balloon up to “15” had a greater likelihood of it popping compared to pumping the balloon up to “5”). After feedback was presented, a new balloon appeared after 1,000 ms. Participants earned one point for every pump of the balloon, and points for all the “win” trials were summed to calculate their total points. Participants were instructed that the goal of the task was to earn as many points as possible.
Face-processing Task
Participants also completed a face-processing task. During this task, participants were shown pictures of different emotional faces (happy, neutral, fear, and anger), as well as other stimuli (e.g., butterflies, houses, and checkerboards). Participants were instructed that the point of the task was to “catch the butterflies” by clicking a button whenever a butterfly appeared on the screen. This instruction was given to keep children and adolescents’ attention during the task; however, our main goal was to investigate face-processing to angry faces and angry compared with neutral faces. Four blocks were included in this task. The angry face was presented 60 times throughout the task. Overall, there were 496 trials: 240 face trials and 256 nonface trials (checkerboards, houses, and butterflies).
Electrophysiological Recording
Electroencephalography (EEG) was recorded continuously from a BioSemi ActiveTwo system using a 96-channel montage and 7 face sensors. The data were digitized at a sampling rate of 512 Hz. Pre-processing was conducted to identify (1)channels/components that were unreliable within a given time-period, (2)time-periods that were unreliable, (3) and channels/components that were unreliable throughout the recording.
Pre-processing (Channels)
Pre-processing was automated (using MATLAB 2012b scripts) to be performed using EEGLAB (Delorme & Makeig, 2004) version 13.6.5b and was then executed using Octave on Compute Canada’s high performance computer cluster (Cedar: see Desjardins & Segalowitz, 2013; Desjardins et al., 2020; van Noordt et al., 2017, van Noordt et al., 2015 for more details). The data were first separated into 1 second nonoverlapping time windows. For each time window, the voltage variance across each channel was calculated (a 20% trimmed mean was used). Channels were flagged as unreliable if they had a z-score six times greater than the voltage variance across all channels. Time-periods (i.e., the 1-second time windows) were considered unreliable if more than 10% of the channels were identified as having extreme voltage variances. Finally, any channels that were flagged in more than 20% of the time-periods were considered unreliable throughout the recording.
The data were re-referenced to an interpolated average of 19 sites, excluding flagged channels. The data were filtered with a 1-Hz high pass and 30-Hz low pass filter given that cortical activity would not be expected to exceed 30 Hz. After this step, the data were again checked for the same issues reported above: (1) channels that are unreliable within a given time-period, (2)time-periods that are unreliable, (3) and channels that are unreliable throughout the recording. Specifically, any channels that were unlike its neighbouring channels (e.g., had a low correlation with channels around it), were flagged. A channel was flagged as unreliable if it had a z-score that was 2.326 times greater than the mean of the 20% trimmed distribution of correlation coefficients. Time-periods were considered unreliable if more than 10% of the channels within the window were flagged as unreliable. Any individual channels that were flagged in more than 10% of time-periods were considered unreliable across the entire recording. Bridged channels (i.e., channels that are highly correlated with invariable signal) were identified after dividing the average maximum correlation by the standard deviation of the distribution of correlation coefficients. Channels that had a positive z-score that was eight times greater than the 40% trimmed distribution of coefficients were flagged as bridged channels.
Pre-Processing (Components)
After pre-processing the channel data, all data that had not been flagged as unreliable were concatenated back into continuous data. These data were then submitted to an initial Adaptive Mixture of Independent Component Analysis (AMICA) to identify different components of the EEG data (e.g., heart rate components, eye blink components, cortical components, etc.). This process helps to separate brain activity (neural components) from non-neural activity (e.g., muscle movement).
During this procedure, the data were windowed into 1 second time epochs. Unreliable components were detected by comparing each individual component to the variance among all components. Components were flagged if they had a z-score that was 2.326 times greater than the trimmed mean. Time-periods that had more than 10% of its components flagged were considered unreliable. The data were then concatenated into the continuous time course and submitted to three simultaneous AMICA decompositions to assess whether components were replicable (i.e., is muscle movement consistently being classified as muscle movement when the process is repeated multiple times). The procedure above for identifying unreliable components (within 1-second epochs) was completed again using the continuous time series data. Next, a dipole (which identifies the position and orientation for the distribution of positive and negative voltages) was fit using the dipfit plugin in Matlab (Oostenveld et al., 2011). Components with a dipole fit residual variance greater than 15% were flagged. Finally, components were classified using the ICMARC plugin. This process assesses each component against a crowd-sourced database to identify activation consistent with five different categories: eye blinks, neural, heart, lateral eye movements, muscle contamination, and mixed signal.
After pre-processing, a manual quality control review was completed to ensure that the decisions made during pre-processing were appropriate. This procedure was completed by one trained research assistant who assessed the accuracy of the independent component classifications. For example, the research assistant would identify whether cortical components were correctly distinguished from non-cortical components (e.g., muscle, eye blinks, etc.) based on topographical projection, continuous activation, dipole fit, and power spectrum profile. Thus, the quality control review involved using the independent components to help with artifact correction.
EEG post-processing
EEG data were then segmented into single trials and time-locked to the onset of the (1)no-go response (and correct response) from the Go/No-Go task, (2) negative feedback (and positive feedback) from the BART task, and (3) angry faces (and neutral faces) from the face-processing task. A final quality check was completed to identify (and remove) channels that had extreme voltage fluctuations (±50 mV). Channels that were removed during pre-processing were interpolated (i.e., rebuilt using the remaining channel data) to the full montage of 103 channels (96 scalp, 7 exogenous) using spherical spline. The current study used frontocentral midline sites (FCz: electrodes A8 and B8 on our montage) to identify the ERN and CRN during the no-go/task and epochs were baseline corrected at −600 to −400. Similar to previous studies (Fein & Chang, 2008; Hassall et al., 2013; Heffer & Willoughby, 2020), the current study used central midline sites (Cz: electrodes A19 and B19 on our montage) to identify the P3 activation during the BART task; epochs were baseline corrected at −200 to 0. Finally, posterior-temporal sites (P7 and P8; electrodes C2, C3, C12, and C13 on our montage) were used to identify the N170 during the face processing task; epochs were baseline corrected at −200 to 0.
Plan of Analysis
We used STATSLAB, an open-source toolbox that implements robust statistics for analysis of EEG data to extract the ERPs for each task (Campopiano et al., 2018). This software allows for testing using percentile bootstrap and trimmed means, a technique that is robust to distribution characteristics such as skew, outliers, uneven tails, and various model assumption violations (Wilcox, 2017).
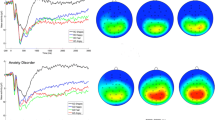
In STATSLAB, single trial data for our channels were extracted and averaged together. For each subject, the single trial data were resampled, with replacement, to generate a surrogate sampling distribution. The 20% trimmed mean was taken across trials, at each time point (i.e., removing the most extreme voltages at each time point), to generate a robust bootstrapped ERP. Iterating this process of resampling, trimming, and scoring the difference wave was performed 1,000 times (see Campopiano et al., 2018 for details). The P3 was extracted at the most positive points (315 ms for losses; 307 ms for wins); all other ERPs were extracted at the most negative points (176 ms for anger N170; 174 ms for neutral N170; 35 ms for ERN; 10 ms for CRN). See Fig. 1 for the ERPs and corresponding topographies.
Waveforms and topographical maps show the ERN and CRN during the go/no-go task (0-100 ms), the P3 to losses and to wins during the BART task (250-400 ms), and the N170 to angry faces and to neutral faces during the face-processing task (140-200 ms). Black dots on topographical maps indicate the channel cluster used for analysis. Of note, the N170 does not cross zero in our sample, due to a large/dominating P1 amplitude; this finding is typical among children and adolescent populations (Kuefner et al., 2010; Taylor et al., 2004). The orange waveforms represent neural activation to threatening situations; the blue waveforms represent neural activation to nonthreatening situations
We next created a variable to identify consistently high neural activation across the tasks. For the BART task, given that the P3 is a positive waveform, scores above the mean reflect having high neural activation to threats. For both the go/no-go task and the face processing task, given that the ERN and the N170 are negative waveforms, scores below the mean reflect having high neural activation to threats. We created a consistency variable, whereby a score of 4 represented having consistently high neural activation on all three ERPs, a score of 3 represented having high neural activation on two out of three tasks, a score of 2 represented having high neural activation on one out of three tasks, and a score of 1 represented having high neural activation on none of the tasks (i.e., low neural activation across all three tasks). Thus, higher scores represent more situations where individuals had high neural activation to threats, whereas lower scores represent less situations where individuals had high neural activation to threats.
In a follow-up analysis, we replicated this analysis using the difference score for each ERP (P3 loss- P3 win; angry N170- neutral N170; ERN- CRN). For the BART task, participants with scores above zero would have a larger P3 amplitude to loss feedback compared with win feedback. For both the go/no-go task and the face processing task, scores below zero reflect having larger ERP activation to the threat (mistakes and angry faces) compared to the nonthreatening situations (successful button presses and neutral faces). In this case, we created a consistency variable whereby a score of 4 represented having consistently higher neural activation to threats than nonthreats on all three ERPs, a score of 3 represented having higher neural activation to threats than nonthreats on two out of three tasks, a score of 2 represented having higher neural activation to threats than nonthreats on one out of three tasks, and a score of 1 represented having higher neural activation to threats than nonthreats on none of the tasks (i.e., lower neural activation to threats than to nonthreats across all three tasks). Thus, a higher difference score reflects more situations in which an individual has higher neural activation to threats than to nonthreats.
Results
Means and standard deviations for all study variables are reported in Table 1. Of interest, the mean score on neural consistency to threats was 2.47 (SD = 0.85), suggesting that on average, participants had high neural activation to threats on one or two tasks. Approximately, 11.8% of participants had consistently high neural activation to all three tasks, 34.8% of participants had high neural activation to two out of three tasks, 41.6% of participants had high neural activation to one out of three tasks, and 11.8% of participants had low neural activation on all three tasks. For the difference score, the average was 2.86 (SD = 0.84); higher scores represented consistently higher neural activation to threats than nonthreats. Approximately, 24.8% of participants had consistently higher neural activation to threats than nonthreats on all three tasks, 41% of participants had higher neural activation to threats than nonthreats on two out of three tasks, 29.8% of participants had higher neural activation to threats than nonthreats on one out of three tasks, and 4.4% of participants had no tasks whether they had higher neural activation to threats than nonthreats.
What factors predict consistently high neural activation to threats?
A linear regression was used investigate what factors (sensitivity to threat, impulsivity, age, pubertal status, sex, and parental education) predict consistently high neural activation to threats. Table 2 contains the results of the linear regression. Sensitivity to threat [β = 0.236, p = 0.017], impulsivity [β = −0.374, p = 0.002], and sex [β = −0.284, p = 0.043] were the only significantly predictors of consistent neural activation to threats. Specifically, higher self-reported threat sensitivity and lower self-reported impulsivity predicted having consistently higher neural activation to threats. Additionally, males had consistently higher neural activation to threats than females.
What factors predict consistently higher neural activation to threats than to nonthreats (i.e., using the difference score)?
A linear regression was used investigate what factors (sensitivity to threat, impulsivity, age, pubertal status, sex, and parental education) predict consistently higher neural activation to threats than nonthreats (i.e., difference score). Table 3 contains the results of the linear regression. Sensitivity to threats [β = 0.208, p = 0.036] was the only significantly predictor of the difference score. Specifically, higher self-reported threat sensitivity predicted having consistently higher neural activation to threats than to nonthreats.
Discussion
Threat sensitivity frequently has been characterized as a risk factor for the development of anxiety (Bar-Haim et al., 2007). Self-report measures of threat sensitivity often combine different threat-related situations (e.g., making a mistake, receiving negative feedback, worrying about someone being angry with you) into one measure. Neuroscience research, on the other hand, often investigates these threat-related situations separately using different tasks. The current study used three EEG tasks to investigate consistency of neural activation to threats.
We were interested in what demographic and self-report factors are associated with consistently high neural activation to threats (e.g., Do adolescents have more consistently high neural activation to threats than children? Do individuals who self-report greater threat sensitivity have consistently high neural activation across these tasks?).
First, we created a measure of neural consistency to threats. Using three different ERPs (P3, N170, and ERN) and three different tasks (the BART, a go/no-go task, a face-processing task), we found that it was quite common for youth to have high neural sensitivity to threats on at least one or two tasks (M = 2.4, SD = 0.85). It was less common, however, to have consistently high neural activation to threats on all three tasks (~12% of the sample). Our results show that although self-report measures of threat sensitivity group these different threat-related events together, not all youth have consistently high neural activation to receiving negative feedback, angry faces, and making mistakes.
Our results show that self-reported impulsivity was a predictor of consistently low neural activation, while self-reported threat sensitivity was a predictor of consistently high neural activation to threats. We also found that males had consistently higher neural activation to threats than females. The latter finding was not part of our original predictions. It is not entirely clear why males would have higher neural consistency to threats than females. Given that this is the first study investigating neural consistency across multiple tasks, future research is needed to tease apart this association.
On the other hand, our findings regarding impulsivity and threat sensitivity were in line with our predictions. Indeed, individuals with high self-reported impulsivity may be less troubled by different types of threats, given that they are more likely to engage in nonreflective, stimulus-driven responding (Nigg, 2017). At the same time, individuals with high self-reported threat sensitivity seem to have higher neural sensitivity to a variety of different threats. Therefore, investigating consistent neural activation across different tasks may be an important way to identify youth who are at the greatest risk for impulsivity and threat sensitivity. Indeed, if individuals have a consistent response across three different threats, it would be more likely that the individuals’ threat sensitivity is being accurately classified. This result is in line with the cumulative risk hypothesis (i.e., a greater number of risk factors is associated with more problem behaviors; Appleyard et al., 2005). Thus, individuals who have consistently high (or low) neural activation across multiple situations would be the most likely to self-report high levels of sensitivity to threat (or impulsivity). Identifying youth with consistent neural activation to different threats may be an important avenue for researchers interested in the development of anxiety. Indeed, given that many youth show high neural activation to at least one threatening event, cumulative neural activation to a variety of different threats may be a promising approach to identify youth who are truly at risk for the development of anxiety.
Surprisingly, we did not find that adolescents (i.e., individuals with more advanced pubertal status or older age) had consistently higher neural activation to threats. We expected that adolescence may be a time of heightened sensitivity to emotionally salient events, such as threatening events (Casey, 2015; Somerville et al., 2010; Steinberg et al., 2008), and therefore, we thought that adolescents would have heightened neural activation across a variety of different threatening events. This nonsignificant finding, however, suggests that children also show consistently high neural sensitive to different threats (see Chong & Meyer, 2019; Heffer & Willoughby, 2020; O’Toole et al., 2013, for other studies showing that children with anxiety-related symptoms can show high ERP activation to these different threatening situations). Thus, youth who demonstrate consistently high neural sensitivity to different threats should be further investigated, especially when considering early interventions aimed at identifying youth who may self-report sensitivity to threats.
In a follow-up analysis, we also wanted to investigate whether our results were consistent when using the difference scores for each ERP (i.e., P3 loss - P3 wins; N170 angry - N170 neutral; ERN - CRN). The main conclusion from our study was replicated when using the difference score: youth with higher self-reported threat sensitivity had consistently higher neural activation to threats than to nonthreats. However, we did not replicate our findings regarding impulsivity and sex. There may be several reasons for the latter findings. In terms of impulsivity, for example, if impulsive individuals are less reflective during the task, they may have been less sensitive to both threatening events and nonthreatening events, providing them with a difference score that thus is similar to others who have high neural activation to both threats and nonthreats. Again, this may be one disadvantage of the difference score—individuals with different patterns of neural activation can end up with the same value on a difference score measure. Additionally, in two out of three of our tasks the nonthreatening event was a positive event (receiving win feedback and making a correct response). Previous research has found that some individuals have high sensitivity to both negative and positive stimuli (Coplan et al., 2006). Thus, individuals who find threatening events aversive (but also find positive events exciting) may not be well represented by a difference score. Future research is needed to replicate these results using multiple threat-related situations in comparison to neutral events.
Our study has important strengths, including a large sample, inclusion of three different ERP tasks, and the use of multiple methods (e.g., self-report and EEG). At the same time, our study is not without limitations. First, we did not include the full scale for either self-report sensitivity to threat or self-reported impulsivity as the data were part of a larger study assessing a wide range of constructs, and it was not feasible to include every item from each scale due to time constraints. Future research would benefit from investigating group differences in threat-sensitivity and impulsivity using the full scales. Second, our study is concurrent; thus, casual inferences cannot be concluded (e.g., we are unable to ascertain whether more consistent neural activation across tasks leads to greater self-reported sensitivity to threat and/or whether greater self-reported sensitivity to threat leads to more consistent neural sensitivity). Longitudinal studies investigating both self-report measures and neural activation at each time point are necessary before drawing these conclusions. Finally, we did not assess whether there were individual differences in specific combinations of tasks (e.g., individuals who have high neural activation to negative feedback, low neural activation to making mistakes, but high neural activation to angry faces). Given that our main interest was in consistent threat sensitivity, this breakdown was not necessary to answer our research question.
Despite these limitations, our study has important implications. A small (~12% of the sample), but important percentage of the sample was identified as having consistently high neural response to threats. Furthermore, consistently higher neural activation to threats (and higher neural activation to threats than to nonthreats) was associated with higher self-reported threat sensitivity. Our results suggest that although it is common for youth to have high neural activation in response to one threat-related tasks, it is far less common for youth to have consistently high neural activation to threats. Threat sensitivity is thought to be associated with the development of anxiety; however, not all youth who are sensitive to threats develop anxiety (Pérez-Edgar et al., 2010). Given that anxiety affects roughly 7–15% of youth (Beesdo et al., 2009; Ghandour et al., 2019), consistency of neural activation to threats may be an important group to investigate in order to identify nonnormative levels of threat sensitivity.
References
Appleyard, K., Egeland, B., van Dulmen, M. H. M., & Sroufe, L. A. (2005). When more is not better: the role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 46(3), 235–245. https://doi.org/10.1111/j.1469-7610.2004.00351.x
Baars, M. A. E., Nije Bijvank, M., Tonnaer, G. H., & Jolles, J. (2015). Self-report measures of executive functioning are a determinant of academic performance in first-year students at a university of applied sciences. Frontiers in Psychology, 6, 1131. https://doi.org/10.3389/fpsyg.2015.01131
Balle, M., Tortella-Feliu, M., & Bornas, X. (2013). Distinguishing youths at risk for anxiety disorders from self-reported BIS sensitivity and its psychophysiological concomitants. International Journal of Psychology: Journal International de Psychologie, 48(5), 964–977. https://doi.org/10.1080/00207594.2012.723804
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1–24. https://doi.org/10.1037/0033-2909.133.1.1
Barratt, E. S. (1959). Anxiety and Impulsiveness Related to Psychomotor Efficiency. Perceptual and Motor Skills, 9(3), 191–198. https://doi.org/10.2466/pms.1959.9.3.191
Batty, M., & Taylor, M. J. (2006). The development of emotional face processing during childhood. Developmental Science, 9(2), 207–220. https://doi.org/10.1111/j.1467-7687.2006.00480.x
Bechor, M., Ramos, M. L., Crowley, M. J., Silverman, W. K., Pettit, J. W., & Reeb-Sutherland, B. C. (2019). Neural Correlates of Attentional Processing of Threat in Youth with and without Anxiety Disorders. Journal of Abnormal Child Psychology, 47(1), 119–129. https://doi.org/10.1007/s10802-018-0424-8
Beesdo, K., Knappe, S., & Pine, D. S. (2009). Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. The Psychiatric Clinics of North America, 32(3), 483–524. https://doi.org/10.1016/j.psc.2009.06.002
Boksem, M. A. S., Tops, M., Kostermans, E., & De Cremer, D. (2008). Sensitivity to punishment and reward omission: evidence from error-related ERP components. Biological Psychology, 79(2), 185–192. https://doi.org/10.1016/j.biopsycho.2008.04.010
Campopiano, A., van Noordt, S. J. R., & Segalowitz, S. J. (2018). STATSLAB: An open-source EEG toolbox for computing single-subject effects using robust statistics. Behavioural Brain Research, 347, 425–435. https://doi.org/10.1016/j.bbr.2018.03.025
Carskadon, M. A., & Acebo, C. (1993). A self-administered rating scale for pubertal development. The Journal of Adolescent Health, 14(3), 190–195. https://doi.org/10.1016/1054-139x(93)90004-9
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. https://doi.org/10.1037//0022-3514.67.2.319
Casey, B. J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. https://doi.org/10.1146/annurev-psych-010814-015156
Chandrakumar, D., Feuerriegel, D., Bode, S., Grech, M., & Keage, H. A. D. (2018). Event-related potentials in relation to risk-taking: A systematic review. Frontiers in Behavioral Neuroscience, 12, 111. https://doi.org/10.3389/fnbeh.2018.00111
Checa, P., Castellanos, M. C., Abundis-Gutiérrez, A., & Rosario Rueda, M. (2014). Development of neural mechanisms of conflict and error processing during childhood: implications for self-regulation. Frontiers in Psychology, 5, 326. https://doi.org/10.3389/fpsyg.2014.00326
Chong, L. J., & Meyer, A. (2019). Understanding the link between anxiety and a neural marker of anxiety (the error-related negativity) in 5 to 7 year-old children. Developmental Neuropsychology, 44(1), 71–87. https://doi.org/10.1080/87565641.2018.1528264
Coplan, R. J., Wilson, J., Frohlick, S. L., & Zelenski, J. (2006). A person-oriented analysis of behavioral inhibition and behavioral activation in children. Personality and Individual Differences, 41(5), 917–927. https://doi.org/10.1016/j.paid.2006.02.019
Davies, P. L., Segalowitz, S. J., & Gavin, W. J. (2004). Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology, 25(3), 355–376. https://doi.org/10.1207/s15326942dn2503_6
De Pascalis, V., Strippoli, E., Riccardi, P., & Vergari, F. (2004). Personality, event-related potential (ERP) and heart rate (HR) in emotional word processing. Personality and Individual Differences, 36(4), 873–891. https://doi.org/10.1016/S0191-8869(03)00159-4
Debener, S., Ullsperger, M., Siegel, M., Fiehler, K., von Cramon, D. Y., & Engel, A. K. (2005). Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(50), 11730–11737. https://doi.org/10.1523/JNEUROSCI.3286-05.2005
Delorme, A., & Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Denefrio, S., Myruski, S., Mennin, D., & Dennis-Tiwary, T. A. (2019). When neutral is not neutral: Neurophysiological evidence for reduced discrimination between aversive and non-aversive information in generalized anxiety disorder. Motivation and Emotion, 43(2), 325–338.
Desjardins, J. A., & Segalowitz, S. J. (2013). Deconstructing the early visual electrocortical responses to face and house stimuli. Journal of Vision, 13(5). https://doi.org/10.1167/13.5.22
Desjardins, J. A., van Noordt, S., Huberty, S., Segalowitz, S. J., & Elsabbagh, M. (2020). EEG Integrated Platform Lossless (EEG-IP-L) pre-processing pipeline for objective signal quality assessment incorporating data annotation and blind source separation. Journal of Neuroscience Methods, 347, 108961. https://doi.org/10.1016/j.jneumeth.2020.108961
Downes, M., Bathelt, J., & De Haan, M. (2017). Event-related potential measures of executive functioning from preschool to adolescence. Developmental Medicine and Child Neurology, 59(6), 581–590. https://doi.org/10.1111/dmcn.13395
DuPuis, D., Ram, N., Willner, C. J., Karalunas, S., Segalowitz, S. J., & Gatzke-Kopp, L. M. (2015). Implications of ongoing neural development for the measurement of the error-related negativity in childhood. Developmental Science, 18(3), 452–468. https://doi.org/10.1111/desc.12229
Eppinger, B., Mock, B., & Kray, J. (2009). Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology, 46(5), 1043–1053. https://doi.org/10.1111/j.1469-8986.2009.00838.x
Ernst, M., Pine, D. S., & Hardin, M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36(3), 299–312. https://doi.org/10.1017/S0033291705005891
Euser, A. S., Evans, B. E., Greaves-Lord, K., Huizink, A. C., & Franken, I. H. A. (2013). Parental rearing behavior prospectively predicts adolescents’ risky decision-making and feedback-related electrical brain activity. Developmental Science, 16(3), 409–427. https://doi.org/10.1111/desc.12026
Fein, G., & Chang, M. (2008). Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug and Alcohol Dependence, 92(1–3), 141–148. https://doi.org/10.1016/j.drugalcdep.2007.07.017
Ghandour, R. M., Sherman, L. J., Vladutiu, C. J., Ali, M. M., Lynch, S. E., Bitsko, R. H., & Blumberg, S. J. (2019). Prevalence and Treatment of Depression, Anxiety, and Conduct Problems in US Children. The Journal of Pediatrics, 206, 256-267.e3. https://doi.org/10.1016/j.jpeds.2018.09.021
Grose-Fifer, J., Migliaccio, R., & Zottoli, T. M. (2014). Feedback processing in adolescence: an event-related potential study of age and gender differences. Developmental Neuroscience, 36(3–4), 228–238. https://doi.org/10.1159/000358917
Gu, R., Zhang, D., Luo, Y., Wang, H., & Broster, L. S. (2018). Predicting risk decisions in a modified Balloon Analogue Risk Task: Conventional and single-trial ERP analyses. Cognitive, Affective & Behavioral Neuroscience, 18(1), 99–116. https://doi.org/10.3758/s13415-017-0555-3
Hajcak, G., & Foti, D. (2008). Errors are aversive: defensive motivation and the error-related negativity. Psychological Science, 19(2), 103–108. https://doi.org/10.1111/j.1467-9280.2008.02053.x
Hajcak, G., McDonald, N., & Simons, R. F. (2003). Anxiety and error-related brain activity. Biological Psychology, 64(1–2), 77–90. https://doi.org/10.1016/S0301-0511(03)00103-0
Hassall, C. D., Holland, K., & Krigolson, O. E. (2013). What do I do now? An electroencephalographic investigation of the explore/exploit dilemma. Neuroscience, 228, 361–370. https://doi.org/10.1016/j.neuroscience.2012.10.040
Heffer, T., & Willoughby, T. (2020). Sensitivity to negative feedback among children and adolescents: An ERP study comparing developmental differences between high-worriers and low-worriers. Cognitive, Affective & Behavioral Neuroscience, 20, 624–635. https://doi.org/10.3758/s13415-020-00791-8
Hileman, C. M., Henderson, H., Mundy, P., Newell, L., & Jaime, M. (2011). Developmental and Individual Differences on the P1 and N170 ERP Components in Children With and Without Autism. Developmental Neuropsychology, 36(2), 214–236. https://doi.org/10.1080/87565641.2010.549870
Hinojosa, J. A., Mercado, F., & Carretié, L. (2015). N170 sensitivity to facial expression: A meta-analysis. Neuroscience and Biobehavioral Reviews, 55, 498–509. https://doi.org/10.1016/j.neubiorev.2015.06.002
Holroyd, C. B., & Yeung, N. (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences, 16(2), 122–128. https://doi.org/10.1016/j.tics.2011.12.008
Huang, W. J., Chen, W. W., & Zhang, X. (2015). The neurophysiology of P 300--an integrated review. European Review for Medical and Pharmacological Sciences, 19(8), 1480–1488.
Humphreys, K. L., Telzer, E. H., Flannery, J., Goff, B., Gabard-Durnam, L., Gee, D. G., Lee, S. S., & Tottenham, N. (2016). Risky decision making from childhood through adulthood: Contributions of learning and sensitivity to negative feedback. Emotion, 16(1), 101–109. https://doi.org/10.1037/emo0000116
Jetha, M. K., Zheng, X., Goldberg, J. O., Segalowitz, S. J., & Schmidt, L. A. (2013). Shyness and emotional face processing in schizophrenia: an ERP study. Biological Psychology, 94(3), 562–574. https://doi.org/10.1016/j.biopsycho.2013.10.001
Johnson, S. L., Turner, R. J., & Iwata, N. (2003). BIS/BAS levels and psychiatric disorder: An Epidemiological Study. Journal of Psychopathology and Behavioral Assessment, 25(1), 25–36. https://doi.org/10.1023/A:1022247919288
Justus, A. N., Finn, P. R., & Steinmetz, J. E. (2001). P300, Disinhibited Personality, and Early-Onset Alcohol Problems. Alcoholism, Clinical and Experimental Research, 25(10), 1457–1466. https://doi.org/10.1111/j.1530-0277.2001.tb02147.x
Katz, B. A., Matanky, K., Aviram, G., & Yovel, I. (2020). Reinforcement sensitivity, depression and anxiety: A meta-analysis and meta-analytic structural equation model. Clinical Psychology Review, 77, 101842. https://doi.org/10.1016/j.cpr.2020.101842
Kim, E. Y., Iwaki, N., Uno, H., & Fujita, T. (2005). Error-related negativity in children: effect of an observer. Developmental Neuropsychology, 28(3), 871–883. https://doi.org/10.1207/s15326942dn2803_7
Kolassa, I.-T., & Miltner, W. H. R. (2006). Psychophysiological correlates of face processing in social phobia. Brain Research, 1118(1), 130–141. https://doi.org/10.1016/j.brainres.2006.08.019
Kolassa, I.-T., Kolassa, S., Musial, F., & Miltner, W. H. R. (2007). Event-related potentials to schematic faces in social phobia. Cognition and Emotion, 21(8), 1721–1744. https://doi.org/10.1080/02699930701229189
Kolassa, I.-T., Kolassa, S., Bergmann, S., Lauche, R., Dilger, S., Miltner, W. H. R., & Musial, F. (2009). Interpretive bias in social phobia: An ERP study with morphed emotional schematic faces. Cognition and Emotion, 23(1), 69–95. https://doi.org/10.1080/02699930801940461
Kuefner, D., de Heering, A., Jacques, C., Palmero-Soler, E., & Rossion, B. (2010). Early Visually Evoked Electrophysiological Responses Over the Human Brain (P1, N170) Show Stable Patterns of Face-Sensitivity from 4 years to Adulthood. Frontiers in Human Neuroscience, 3, 67. https://doi.org/10.3389/neuro.09.067.2009
Ladouceur, C. D., Dahl, R. E., Birmaher, B., Axelson, D. A., & Ryan, N. D. (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(10), 1073–1082. https://doi.org/10.1111/j.1469-7610.2006.01654.x
Lejuez, C. W., Read, J. P., Kahler, C. W., Richards, J. B., Ramsey, S. E., Stuart, G. L., Strong, D. R., & Brown, R. A. (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). Journal of Experimental Psychology. Applied, 8(2), 75–84. https://doi.org/10.1037//1076-898X.8.2.75
Luck, S. J. (2005). An introduction to event related potentials and their neural origins. An Introduction to the Event Related Potential Technique, 11. https://ci.nii.ac.jp/naid/10030430963/
Mathalon, D. H., Whitfield, S. L., & Ford, J. M. (2003). Anatomy of an error: ERP and fMRI. Biological Psychology, 64(1–2), 119–141. https://doi.org/10.1016/s0301-0511(03)00105-4
McCormick, E. M., & Telzer, E. H. (2017). Failure to retreat: Blunted sensitivity to negative feedback supports risky behavior in adolescents. NeuroImage, 147, 381–389. https://doi.org/10.1016/j.neuroimage.2016.12.041
Meyer, A. (2017). A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience, 27, 58–68. https://doi.org/10.1016/j.dcn.2017.08.001
Meyer, A., & Hajcak, G. (2019). A review examining the relationship between individual differences in the error-related negativity and cognitive control. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 144, 7–13. https://doi.org/10.1016/j.ijpsycho.2019.07.005
Miltner, W. H. R., Trippe, R. H., Krieschel, S., Gutberlet, I., Hecht, H., & Weiss, T. (2005). Event-related brain potentials and affective responses to threat in spider/snake-phobic and non-phobic subjects. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 57(1), 43–52. https://doi.org/10.1016/j.ijpsycho.2005.01.012
Nigg, J. T. (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 58(4), 361–383. https://doi.org/10.1111/jcpp.12675
O’Brien, S. F., & Bierman, K. L. (1988). Conceptions and perceived influence of peer groups: interviews with preadolescents and adolescents. Child Development, 59(5), 1360–1365. https://doi.org/10.2307/1130498
O’Toole, L. J., DeCicco, J. M., Berthod, S., & Dennis, T. A. (2013). The N170 to angry faces predicts anxiety in typically developing children over a two-year period. Developmental Neuropsychology, 38(5), 352–363. https://doi.org/10.1080/87565641.2013.802321
Olvet, D. M., & Hajcak, G. (2009). Reliability of error-related brain activity. Brain Research, 1284, 89–99. https://doi.org/10.1016/j.brainres.2009.05.079
Oostenveld, R., Fries, P., Maris, E., & Schoffelen, J.-M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. https://doi.org/10.1155/2011/156869
Pailing, P. E., Segalowitz, S. J., Dywan, J., & Davies, P. L. (2002). Error negativity and response control. Psychophysiology, 39(2), 198–206. https://doi.org/10.1017/S0048577202010247
Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. https://www.ncbi.nlm.nih.gov/pubmed/8778124
Pérez-Edgar, K., Bar-Haim, Y., McDermott, J. M., Chronis-Tuscano, A., Pine, D. S., & Fox, N. A. (2010). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion, 10(3), 349–357. https://doi.org/10.1037/a0018486
Pérez-Edgar, K., Reeb-Sutherland, B. C., McDermott, J. M., White, L. K., Henderson, H. A., Degnan, K. A., Hane, A. A., Pine, D. S., & Fox, N. A. (2011). Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology, 39(6), 885–895. https://doi.org/10.1007/s10802-011-9495-5
Petersen, A. C., Crockett, L., Richards, M., & Boxer, A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. https://doi.org/10.1007/BF01537962
Pleskac, T. J., Wallsten, T. S., Wang, P., & Lejuez, C. W. (2008). Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Experimental and Clinical Psychopharmacology, 16(6), 555–564. https://doi.org/10.1037/a0014245
Reeb-Sutherland, B. C., Vanderwert, R. E., Degnan, K. A., Marshall, P. J., Pérez-Edgar, K., Chronis-Tuscano, A., Pine, D. S., & Fox, N. A. (2009). Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50(11), 1365–1372. https://doi.org/10.1111/j.1469-7610.2009.02170.x
Rossignol, M., Philippot, P., Douilliez, C., Crommelinck, M., & Campanella, S. (2005). The perception of fearful and happy facial expression is modulated by anxiety: an event-related potential study. Neuroscience Letters, 377(2), 115–120. https://doi.org/10.1016/j.neulet.2004.11.091
Ruchsow, M., Spitzer, M., Grön, G., Grothe, J., & Kiefer, M. (2005). Error processing and impulsiveness in normals: evidence from event-related potentials. Brain Research. Cognitive Brain Research, 24(2), 317–325. https://doi.org/10.1016/j.cogbrainres.2005.02.003
Ruchsow, M., Groen, G., Kiefer, M., Buchheim, A., Walter, H., Martius, P., Reiter, M., Hermle, L., Spitzer, M., Ebert, D., & Falkenstein, M. (2008). Response inhibition in borderline personality disorder: event-related potentials in a Go/Nogo task. Journal of Neural Transmission, 115(1), 127–133. https://doi.org/10.1007/s00702-007-0819-0
Sadeh, B., Podlipsky, I., Zhdanov, A., & Yovel, G. (2010). Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Human Brain Mapping, 31(10), 1490–1501. https://doi.org/10.1002/hbm.20952
Santesso, D. L., Segalowitz, S. J., & Schmidt, L. A. (2006). Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science, 9(5), 473–481. https://doi.org/10.1111/j.1467-7687.2006.00514.x
Schafer, J. L., & Graham, J. W. (2002). Missing data: our view of the state of the art. Psychological Methods, 7(2), 147–177. https://doi.org/10.1037//1082-989X.7.2.147
Somerville, L. H., Jones, R. M., & Casey, B. J. (2010). A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. https://doi.org/10.1016/j.bandc.2009.07.003
Stahl, J., & Gibbons, H. (2007). Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: An electrophysiological investigation using a stop-signal task. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(3), 581–596. https://doi.org/10.1016/j.clinph.2006.10.023
Steinberg, L., Albert, D., Cauffman, E., Banich, M., Graham, S., & Woolard, J. (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology, 44(6), 1764–1778. https://doi.org/10.1037/a0012955
Takács, Á., Kóbor, A., Janacsek, K., Honbolygó, F., Csépe, V., & Németh, D. (2015). High trait anxiety is associated with attenuated feedback-related negativity in risky decision making. Neuroscience Letters, 600, 188–192. https://doi.org/10.1016/j.neulet.2015.06.022
Taylor, M. J., Batty, M., & Itier, R. J. (2004). The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience, 16(8), 1426–1442. https://doi.org/10.1162/0898929042304732
Taylor, J. B., Visser, T. A. W., Fueggle, S. N., Bellgrove, M. A., & Fox, A. M. (2018). The error-related negativity (ERN) is an electrophysiological marker of motor impulsiveness on the Barratt Impulsiveness Scale (BIS-11) during adolescence. Developmental Cognitive Neuroscience, 30, 77–86. https://doi.org/10.1016/j.dcn.2018.01.003
Torrubia, R., Ávila, C., Moltó, J., & Caseras, X. (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. https://doi.org/10.1016/S0191-8869(00)00183-5
Van der Elst, W., Ouwehand, C., van der Werf, G., Kuyper, H., Lee, N., & Jolles, J. (2012). The Amsterdam Executive Function Inventory (AEFI): psychometric properties and demographically corrected normative data for adolescents aged between 15 and 18 years. Journal of Clinical and Experimental Neuropsychology, 34(2), 160–171. https://doi.org/10.1080/13803395.2011.625353
van Dinteren, R., Arns, M., Jongsma, M. L. A., & Kessels, R. P. C. (2014). P300 development across the lifespan: a systematic review and meta-analysis. PloS One, 9(2), e87347. https://doi.org/10.1371/journal.pone.0087347
van Noordt, S. J. R., Desjardins, J. A., & Segalowitz, S. J. (2015). Watch out! Medial frontal cortex is activated by cues signaling potential changes in response demands. NeuroImage, 114, 356–370. https://doi.org/10.1016/j.neuroimage.2015.04.021
van Noordt, S. J. R., Desjardins, J. A., Gogo, C. E. T., Tekok-Kilic, A., & Segalowitz, S. J. (2017). Cognitive control in the eye of the beholder: Electrocortical theta and alpha modulation during response preparation in a cued saccade task. NeuroImage, 145(Pt A), 82–95. https://doi.org/10.1016/j.neuroimage.2016.09.054
Vervoort, L., Wolters, L. H., Hogendoorn, S. M., De Haan, E., Boer, F., & Prins, P. J. M. (2010). Sensitivity of Gray’s behavioral inhibition system in clinically anxious and non-anxious children and adolescents. Personality and Individual Differences, 48(5), 629–633. https://doi.org/10.1016/j.paid.2009.12.021
Weinberg, A., Olvet, D. M., & Hajcak, G. (2010). Increased error-related brain activity in generalized anxiety disorder. Biological Psychology, 85(3), 472–480. https://doi.org/10.1016/j.biopsycho.2010.09.011
Wiersema, J. R., van der Meere, J. J., & Roeyers, H. (2007). Developmental changes in error monitoring: an event-related potential study. Neuropsychologia, 45(8), 1649–1657. https://doi.org/10.1016/j.neuropsychologia.2007.01.004
Wieser, M. J., Pauli, P., Reicherts, P., & Mühlberger, A. (2010). Don’t look at me in anger! Enhanced processing of angry faces in anticipation of public speaking. Psychophysiology, 47(2), 271–280. https://doi.org/10.1111/j.1469-8986.2009.00938.x
Wilcox, R. (2017). Introduction to Robust Estimation and Hypothesis Testing Introduction to Robust Estimation and Hypothesis Testing, 4th edn. Academic Press.
Yau, Y. H. C., Potenza, M. N., Mayes, L. C., & Crowley, M. J. (2015). Blunted feedback processing during risk-taking in adolescents with features of problematic Internet use. Addictive Behaviors, 45, 156–163. https://doi.org/10.1016/j.addbeh.2015.01.008
Author information
Authors and Affiliations
Corresponding author
Additional information
Open Practices Statement
None of the data for the current study is available, and this study was not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heffer, T., Willoughby, T. Investigating the consistency of ERPs across threatening situations among children and adolescents. Cogn Affect Behav Neurosci 22, 328–340 (2022). https://doi.org/10.3758/s13415-021-00957-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00957-y