Abstract
Nicotine increases the output of every major neurotransmitter. In previous studies designed to identify the secondary neurotransmitter systems mediating nicotine’s attention-enhancing effects in a rat model, the β-adrenoceptor antagonist propranolol blocked these effects. The present study was designed to test whether this mechanism held true in humans, thus guiding development of novel nicotinic agonists for cognitive enhancement. Twenty-six nonsmokers completed a nicotine (7 mg/24 h transdermally) x propranolol (40 mg p.o., body weight-adjusted) interaction study. Over four test days, each participant received double-placebo, nicotine only, propranolol only, and nicotine plus propranolol in randomized sequence before cognitive testing. No drug effects were seen in a visuospatial attention task. In the Rapid Visual Information Processing Task, performed in two 15-min blocks, neither drug alone significantly affected hit rate, but both drugs combined acted synergistically to alleviate its decrement over time in the first block and displayed additive beneficial effects in the second. In a change detection task, propranolol enhanced accuracy and reduced reaction time independent of nicotine presence. Propranolol also enhanced subjective self-reports of vigor. Overall, the findings were contrary to those hypothesized. Propranolol displayed beneficial effects on cognition, especially on sustaining performance over time. β-adrenoceptor activation by nicotine-induced noradrenaline release appeared to limit performance-enhancing effects of nicotine, because they were unmasked by β-adrenoceptor antagonism. The results suggest that cognitive effects of changes in β-adrenoceptor tone are context-dependent; contrary to rodent paradigms, human cognitive paradigms require no physical orienting in space but prolonged periods of remaining stationary while sustaining predictable processing demands.
Similar content being viewed by others
Introduction
Cognitive benefits of the prototypical non-selective nicotinic acetylcholine receptor (nAChR) agonist nicotine are well established, particularly on processes of attention, but also on sensory and mnemonic processes (Hahn, 2015; Heishman, Kleykamp, & Singleton, 2010; Newhouse, Potter, Dumas, & Thiel, 2011). These effects have been hypothesized to benefit conditions marked by cognitive deficits and nAChR hypofunction, such as Alzheimer’s disease and schizophrenia (Adams & Stevens, 2007; Hong et al., 2011; Kendziorra et al., 2011; Perry et al., 2000; Petrovsky et al., 2010; Wing, Wass, Soh, & George, 2012). This has motivated efforts to develop agonists selective for subtypes of the nAChR with preserved cognitive benefits but fewer unwanted side effects. The focus has been on agonists selective for the α4β2 and the α7 nAChRs—the two most widely expressed nAChR subtypes in the brain. However, although beneficial effects were observed, they generally were of small magnitude and uncertain clinical significance (Haydar & Dunlop, 2010; Hurst, Rollema, & Bertrand, 2013; Radek, Kohlhaas, Rueter, & Mohler, 2010).
Nicotine administration potentiates the cortical and subcortical release of acetylcholine, dopamine, noradrenaline, 5-HT, histamine, GABA, glutamate, and glycine via action at pre- and postsynaptic nAChRs (Lopez, Arce, Vicente, Oset-Gasque, & Gonzalez, 2001; MacDermott, Role, & Siegelbaum, 1999; Role & Berg, 1996; Rollema et al., 2009; Wonnacott, Barik, Dickinson, & Jones, 2006). Despite overlap, these systems differ in the nAChR subtypes that they express; thus, they could be modulated with some degree of selectivity by subtype-selective ligands. Identifying the system(s) central to the cognitive-enhancing effects of nicotine would channel drug development efforts toward the specific nAChR subtypes modulating them.
Preclinical studies have aimed at pinpointing these secondary target system(s). A series of systemic interaction studies employing rat operant paradigms have tested whether the attention-enhancing effects of nicotine could be reversed by dopaminergic, noradrenergic, glutamatergic, or serotonergic antagonists at doses that do not affect performance when given in isolation (Hahn, Shoaib, & Stolerman, 2002a; Hahn & Stolerman, 2005; Quarta, Naylor, Glennon, & Stolerman, 2012; Quarta et al., 2007; Rezvani, Caldwell, & Levin, 2005). Neither a D1- or D2-type dopamine receptor antagonist, nor the α1, α2B, and α2C adrenoceptor antagonist prazosin reduced the attention-enhancing effects of nicotine in the 5-Choice Serial Reaction Time Task (5-CSRTT) at doses that blocked speed-related effects of nicotine (Hahn et al., 2002a; Hahn & Stolerman, 2005). However, the attention-enhancing effects of nicotine were reversed by the β-adrenoceptor antagonist propranolol at doses that had no effect when given without nicotine. Specifically, propranolol blocked the beneficial effects of nicotine on response accuracy and omission errors in the 5-CSRTT. These findings suggested that nicotine-induced noradrenaline release, acting via β-adrenoceptors, is involved in mediating the attention-enhancing effects of nicotine.
A role of noradrenergic neurotransmission in cognitive processes is well established. Tonic firing of locus coeruleus (LC) noradrenergic projections appears to be associated with exploratory behavior, attention shifting, and adjustment to environmental volatility and new behavioral demands, whereas low tonic LC firing accentuates phasic responsiveness to task-relevant stimuli, reflecting a more exclusive focus on stimuli that are currently attended (Aston-Jones & Cohen, 2005). Phasic activation of LC neurons tends to be elicited by stimuli that are salient, unpredictable, or behaviorally significant, and is accompanied by behavioral orienting and good signal discrimination ability (Aston-Jones, Chiang, & Alexinsky, 1991; Aston-Jones, Rajkowski, Kubiak, & Alexinsky, 1994). An optimal range of noradrenaline levels may exist because both very high and very low tonic LC activity weakened phasic responsiveness to targets and increased false alarms (Usher, Cohen, Servan-Schreiber, Rajkowski, & Aston-Jones, 1999).
The purpose of the present study was to test whether nicotine-induced noradrenaline release and activation of β-adrenoceptors is involved in mediating the attention-enhancing effects of nicotine in humans, too. If confirmed, this mechanism of action could guide the search for novel subtype-selective nAChR agonists with cognitive-enhancing properties by directing it toward nAChR subtypes expressed on or afferent to noradrenergic neurons, especially in β-adrenoceptor-expressing systems. Employing a 2 x 2 factorial design, we tested the effects of nicotine versus placebo on cognitive task performance in the presence and absence of propranolol. We hypothesized that propranolol would attenuate the performance-enhancing effects of nicotine.
Methods
Participants
Of 35 healthy nonsmokers randomized for the study, 29 completed it (17 females; 13 African American, 16 Caucasian). Reasons for noncompletion were adverse effects consisting of nausea and vomiting in two cases (both occurred in the Nicotine+Propranolol Session but before ingesting propranolol), no longer meeting inclusion criteria in one case, and withdrawal for personal reasons in three cases. None of these participants completed more than one session; thus, their data were not included in analyses. Data from one completer were excluded because this individual later reported not swallowing the study capsule on one of the test days. Data from two additional completers were excluded from analyses because on at least one of the four test days, their performance was close to random and marked by excessive no-response trials, suggesting a lack of task engagement, i.e., participants were not actually trying to perform the cognitive operations tapped by the tasks. Our target had been at least 24 completers. Power calculation (G*Power) indicates that an interaction of medium effect size (f = 0.286) is detectable in a within-subject design with the 26 completers achieved.
The 26 subjects with valid data were aged 21-53 years (mean ± SD: 33.8 ± 10.9) with 12-19 years of education (15.3 ± 2.3). Participants were recruited from the local community through internet advertising, flyers, and referrals, and gave written informed consent for a protocol approved by the University of Maryland Baltimore Institutional Review Board. Participants had had no more than 40 cigarettes in their lifetime and no nicotine exposure in the past year. Use of centrally active medications, cardiovascular drugs, or any medication that could adversely interact with propranolol; pregnancy; history of neurological or psychiatric disorders, including drug abuse; significant liver or kidney impairment; heart problems; hyper- or hypotension; asthma; chronic obstructive pulmonary disease (COPD); diabetes; history of anaphylactic reactions; and learning disability were exclusion criteria.
Drugs
Nicotine patches were over-the-counter Nicoderm CQ patches (GlaxoSmithKline, Brentford, Middlesex, UK) releasing 7 mg of nicotine in 24 h—the lowest dose available commercially. Placebo patches were generated using AquaHeal Hydrogel Bandages (Spenco Medical Corporation), cut to size and with identifying labeling removed. The hydrogel bandages closely resemble the nicotine patch in color and consistency. The nicotine or size-matched placebo patch was placed on the inside of an adhesive bandage on the day of the study and sealed in a small Ziplock bag until application. The adhesive bandage with the inserted patch was applied by a study nurse not involved in any other study procedures.
USP-grade propranolol HCl powder was obtained from Medisca Inc. (Plattsburgh, NY). Propranolol and matching placebo capsules were generated by a compounding pharmacist at the Maryland Psychiatric Research Center. The inactive filler was lactose. The dose of propranolol administered was adjusted based on the participant’s body weight: 30 mg p.o. was administered to participants weighing <140 lb, 40 mg to participants weighing 140-180 lb, and 50 mg to participants weighing >180 lb.
Study design and procedures
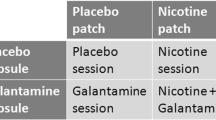
The study adopted a double-blind within-subject design. Each participant completed four test sessions, on separate days, scheduled with at least two intermediate days to ensure complete drug wash-out between sessions. On each test day, a skin patch was applied and a capsule was administered. On one day, both the patch and the capsule were a placebo (Placebo session). On another day, the patch was a nicotine patch and the capsule was a placebo (Nicotine session); on another, the patch was a placebo and the capsule contained propranolol (Propranolol session); and on another day, the patch was a nicotine patch and the capsule contained propranolol (Nicotine+Propranolol session). Thus, the four conditions followed a 2 x 2 factorial design. The sequence in which the drug conditions were tested was counterbalanced across participants to the degree possible (with 29 completers, it was not possible to employ all 24 possible sequences the same number of times). Only the statistician performing the randomization and the dispensing pharmacist were aware of the sequences.
The study involved six total visits: one consent and screening visit, one training visit, and the four test sessions. Screening included a medical history and physical examination, an electrocardiogram, blood and urine labs, a vision test, and tests for drug use, smoking, and pregnancy. During the training visit, participants were given task instructions and performed a full-length version of each of the cognitive tasks described below, to minimize practice effects between test sessions.
Each of the four test sessions took approximately 7 h. Upon arrival in the morning, participants were tested for fever and recent alcohol use or smoking, and a urine sample was tested for pregnancy and drug use, all of which had to be negative for the session to proceed. Baseline measurements were then taken for resting blood pressure and heart rate, as well as a side effects checklist on which participants rated possible adverse effects of nicotine and propranolol (restlessness, weakness/fatigue, dizziness, visual disturbances, tingly hands, nausea, abdominal pain, sweating, palpitations, jitteriness, sleepiness, diarrhea, decreased appetite, stomach discomfort, sore throat, difficulty breathing, headache) as none (1), mild (2), moderate (3), or severe (4). Participants then completed the Profile of Mood States (POMS), an adjective rating questionnaire considered a standardized subjective mood state inventory (McNair, Lorr, & Droppleman, 1971).
Next, the study patch was administered. Vital signs and the side effects checklist were obtained hourly thereafter. During the 5-hour drug-absorption period, participants were permitted to read, watch movies, or use the internet. Three hours after patch administration (always shortly after lunch, which was of the participant’s choosing), participants swallowed the study capsule. From then on, vital signs and the side effects checklist were obtained every 30 min. Five hours after patch application (2 hours after capsule administration), the POMS was again completed, and cognitive testing began. This timing was based on peak drug concentrations after administration reported in the literature. Nicotine plasma concentrations reach asymptote by 5 hours post-patch administration, after which they remain stable, creating an extended testing period despite nicotine’s short half-life (Fant, Henningfield, Shiffman, Strahs, & Reitberg, 2000; Gupta, Benowitz, Jacob, Rolf, & Gorsline, 1993). Propranolol plasma concentrations reach Cmax on average 2.5 hours after p.o. administration (Sharoky, Perkal, Turner, & Lesko, 1988). The order of the cognitive tasks was fixed: first the Spatial Attentional Resource Allocation Task, then the Rapid Visual Information Processing Task, and last the Change Detection Task (see below). Testing was completed in approximately 1.5 hours. Vital signs were measured after the first task. Immediately after cognitive testing, the POMS and side effects checklist were completed, vital signs were taken one last time, and a 5-ml blood sample was obtained from a forearm vein for analysis of nicotine concentrations.
Equipment
All tasks were performed on a 19-in 5:4 IPS LCD monitor with a screen resolution of 1,280 x 1,024, and a refresh rate of 60 Hz. Responses were recorded using a Logitech F310 gamepad controller. Only the left and right bumper buttons were used. In tasks involving a single button, subjects responded with their dominant hand. All tasks were created and run in E-Prime version 2.0.
Task paradigms
The three paradigms were chosen to cover different attentional domains and short-term/working memory (for greater detail, see Hahn et al., 2020; Yuille, Olmstead, Wells, & Hahn, 2017).
Spatial Attentional Resource Allocation Task
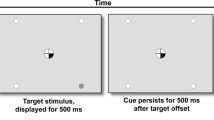
The Spatial Attentional Resource Allocation Task (SARAT) is a Posner-type visuospatial stimulus detection paradigm (Hahn, Ross, & Stein, 2006), in which performance-enhancing effects of nicotine have previously been reported (Hahn et al., 2007; Hahn et al., 2013). Participants fixated on a quartered circle in the center of the screen, black against a light-grey background. They were instructed to respond as quickly as possible when detecting a 500-ms target stimulus appearing in one of four locations in the corners of the screen. The target locations were marked by circular place holders and positioned at ~10° of visual angle. A cue in the central circle preceded the target signal by 400, 700, 1,000, or 1,300 ms, chosen randomly. Either one quarter turned black to indicate the location of the upcoming target (predictive cue), or all four quarters turned black (nonpredictive cue). Predictive cue trials allowed for a narrow attentional focus, whereas nonpredictive cue trials required attention to be spread widely to encompass the entire display. Participants responded with their dominant index finger upon detecting a target. Targets consisted of a placeholder circle filling with a grey and white checkerboard pattern of 3 x 3 pixels each. The cue persisted for 500 ms after target offset. Only task background was then displayed for a variable intertrial interval of 400, 700, 1,000, or 1,300 ms. The task was presented in eight 5-min blocks of 60 trials each: 30 predictive cue trials, of which 6 had no target to discourage anticipatory responding to the cue, and 30 nonpredictive cue trials, of which 6 had no target. To increase the temporal jitter and augment stimulus detection demands, 30 additional 2.7-s periods during which only task background was presented were interspersed randomly between trials. Total task duration was approximately 45 min.
Rapid visual information processing task
The Rapid Visual Information Processing Task (RVIPT) requires the maintenance of intense rapid information processing and working memory demands over time. Performance therefore reflects processing speed, sustained attention, and working memory. A critical aspect of the RVIPT is a performance decrement with time on task, which captures the essence of the concept of sustained attention. The RVIPT has been used extensively to evaluate effects of nicotine on sustained attention (Foulds et al., 1996; Warburton & Mancuso, 1998; Wesnes & Warburton, 1984). The task consists of a string of digits (1 through 9), presented one at a time at a rate of 100/min. Each digit was presented for 600 ms, with no interstimulus interval. Participants were instructed to respond with their dominant index finger when they identified three consecutive odd or even digits. Responses within a 1,800-ms time window following the onset of the last digit of a target sequence were considered hits, all other responses were considered false alarms. On average 8 target sequences were presented per minute. The task was performed in two blocks of 15 min each, with a short (<1 min) break between to ensure feasibility.
Change detection task
The Change Detection Task (CDT) is a visual short-term memory task (Luck & Vogel, 1997) and was included as a probe for potential mnemonic drug effects. A 220-ms encoding array of colored squares was presented. Possible colors were red, magenta, purple, yellow, white, blue, cyan, green, olive, and teal. Half of the trials showed five colored squares, and the other half showed one colored square. After a 1,100-ms retention interval, one square reappeared for 2000 ms, and participants determined whether this square was of the same or a different color than the square previously displayed at this location. On half the trials, the color was the same, on the other half, the color changed. Participants responded “same” with a right button press and “different” with a left button press. Trials were separated by a 1,000-ms intertrial interval. The task consisted of 180 total trials, presented over 5 blocks of 36 trials each. Blocks were separated by short breaks. Total task duration was 13 min.
Blood analyses
Immediately after the blood draw, the sample was centrifuged to separate plasma from red blood cells. Plasma samples were frozen at −80 °C until analysis at study completion.Analyses of nicotine and cotinine plasma concentrations were performed by NMS Labs (Willow Grove, PA) by High Performance Liquid Chromatography/TandemMass Spectrometry (LC-MS/MS). Reporting limits were 2.5 ng/ml for nicotine and 5 ng/ml for cotinine. Because smoking abstinence was considered sufficiently verified, only samples from the Nicotine session and the Nicotine+Propranolol session were analyzed.
Statistical analysis
Vital signs and each subjective state scale from the side effects checklist were analyzed by three-factor ANOVA with nicotine, propranolol, and time as within-subject factors, including only the last five measurement time points at which some absorption of nicotine and propranolol (if administered) had taken place: 4 h and 4:30 h after patch administration (1 h and 1:30 h after capsule administration); 5 h post-patch (2 h post-capsule, start of testing); 5:45 h post-patch (2:45 h post-capsule, mid-testing); and 6:30 h post-patch (3:30 h post-capsule, post-testing).
Each of the seven POMS scales (tension/anxiety, depression, anger/hostility, vigor/activity, fatigue, confusion, and total mood disturbance—a summary index of ratings on all other scales) was analyzed by three-factor ANOVA with nicotine, propranolol, and time (baseline, pre-testing, post-testing) as within-subject factors.
SARAT: Mean RT and the percentage of omission errors were analyzed by separate three-factor ANOVAs with nicotine, propranolol, and cue type (predictive vs. nonpredictive) as within-subject factors.
RVIPT: Because of the large number of non-target events, the false alarm rate (percentage of nontarget events with a target response) was <1% for all but one participant. Expectably, analysis of the false alarm rate did not yield any significant effects, and the sensitivity index A' (Grier, 1971) yielded a virtually identical performance pattern across conditions as the hit rate. Analyses reported here thus focus on the hit rate (percentage of target detections out of all targets presented) and mean RT. These variables were analyzed by four-factor ANOVA with nicotine, propranolol, block (first vs. second 15-min block), and period (three 5-min periods within each block) as within-subject factors.
CDT: Trials without a response were rare, averaging 0.17% ± 0.77 (SD) of trials, and were excluded from analyses. Accuracy (percentage of correct responses out of all response trials) and mean RT were analyzed by separate three-factor ANOVAs with nicotine, propranolol, and set size (1 vs. 5) as within-subject factors.
Effect sizes are reported as partial eta squared (ηp2), with ηp2 = 0.06 generally considered a medium and ηp2 > 0.14 a large effect size (Cohen, 1988). Significance testing was based on p < 0.05, two-sided. All analyses were done using SPSS version 25.
Results
Nicotine and cotinine concentrations in blood plasma
We were unable to obtain blood from two participants. One subject’s nicotine plasma concentration was below the 2.5-ng/ml detection threshold in the Nicotine+Propranolol session despite a high cotinine concentration. In the remaining 23 subjects, nicotine concentrations averaged 6.38 ± 1.90 ng/ml (range 4-12) in the Nicotine Session and 6.26 ± 1.90 ng/ml (range 4-11) in the Nicotine+Propranolol Session, t(22) = 0.38, p = 0.71 (paired t-test), comparable to plasma concentrations observed in past studies, testing smokers and nonsmokers, with a nicotine patch of the same dose (Gorsline, Gupta, Dye, & Rolf, 1993; Hahn et al., 2020). Cotinine concentrations averaged 26.96 ± 10.25 ng/ml in the Nicotine session and 25.84 ± 11.16 ng/ml in the Nicotine+Propranolol session, t(23) = 0.61, p = 0.55.
Vital signs and subjective side effects
The top panels of Figure 1 show that nicotine increased and propranolol decreased systolic and diastolic blood pressure relative to placebo, as confirmed by significant main effects of nicotine and propranolol on both measures, all Fs(1,25) > 12.6, ps < 0.002. These effects appeared to cancel each other out when both drugs were administered in combination. Blood pressure tended to decrease over time. For systolic blood pressure, this was confirmed by a main effect of time, F(4,100) = 6.10, p < 0.001. For diastolic blood pressure, there was no significant main effect of time, F(4,100) = 1.24, p = 0.30, probably because nicotine counteracted this decline [nicotine x time: F(4,100) = 3.38, p = 0.012].
Drug effects on vital signs and self-reports of nausea from the side effects checklist. Averages are shown from the last five measurement time points. Possible ratings on the self-report scales were 1 = none, 2 = mild, 3 = moderate, and 4 = severe. Error bars reflect SEMs, adjusted to remove variability in the average performance across drug conditions (Cousineau, 2007; Morey, 2008)
Propranolol lowered heart rate by over 9 bpm on average (Figure 1, bottom left), giving rise to a significant main effect of propranolol, F(1,25) = 66.2, p < 0.001. Nicotine slightly raised heart rate as confirmed by a main effect of nicotine, F(1,25) = 4.86, p = 0.037, but did not counteract the effects of propranolol. There were no significant interactions.
From among the subjective side effect items, significant drug effects were seen on nausea (Figure 1, bottom right panel) in the form of a nicotine x time interaction, F(4,100) = 3.71, p = 0.007, ηp2 = 0.13. This was based on four participants reporting nausea (all “mild”) during or after cognitive testing in one of the sessions involving nicotine administration. Furthermore, there was a significant main effect of nicotine on sleepiness, F(1,25) = 4.95, p = 0.035, ηp2 = 0.17, consisting of less sleepiness in sessions involving nicotine administration (data not shown). No other subjective side effect items showed any drug effects.
Subjective state as measured by the POMS
The POMS revealed significant worsening of mood over time, as confirmed by main effects of time on the tension, F(2,50) = 6.30, p = 0.004, ηp2 = 0.20, vigor, F(2,50) = 8.48, p = 0.001, ηp2 = 0.25, fatigue, F(2,50) = 3.56, p = 0.036, ηp2 = 0.13, and total mood disturbance scale, F(2,50) = 5.36, p = 0.008, ηp2 = 0.18, especially from pre- to post-testing, but for tension and vigor also over the course of the absorption period. The main effect of nicotine and the nicotine x time interaction were not significant on any scale, all ps > 0.35. However, there was a significant main effect of propranolol on vigor, F(1,25) = 5.87, p = 0.023, ηp2 = 0.19, and a significant propranolol x time interaction on tension, F(2,50) = 3.71, p = 0.031, ηp2 = 0.13. Figure 2 shows effects of propranolol collapsed over the presence and absence of nicotine. The left panel illustrates that propranolol increased self-reports of vigor and appeared to alleviate its decline over time, although the propranolol x time interaction was not significant, F(2,50) = 1.16, p = 0.32, ηp2 = 0.04. The right panel of Figure 2 shows that propranolol reversed the increase in tension over the course of the absorption period (i.e., from baseline to pre-testing), but not the increase from pre- to post-testing. There were no nicotine x propranolol interactions [all ps > 0.4, ηp2 < 0.03, except fatigue, p = 0.10, ηp2 = 0.10].
Effects of propranolol on the vigor and tension scales of the Profile Of Mood States. Measurements at three time points are shown, averaged over the presence and absence of nicotine. Error bars reflect SEMs, adjusted to remove between-subject variability in the average performance across drug conditions (Cousineau, 2007; Morey, 2008). **p < 0.01, paired t-test
Drug effects on task performance
SARAT
Significant main effects of cue type confirmed that RT was slower, F(1,25) = 18.0, p < 0.001, and omission errors were higher, F(1,25) = 5.43, p = 0.028, in nonpredictive than predictive cue trials. Main effects or interactions involving either of the drugs did not reach significance.
RVIPT
Hit rate
A significant main effect of time period, F(2,50) = 36.0, p < 0.001, ηp2 = 0.59, reflected performance decrement over time within each block. This decrement was less pronounced over the course of the second block [period x block interaction F(2,50) = 4.38, p = 0.018, ηp2 = 0.15], which started off at a lower performance level than the first block in most conditions. Nicotine increased hit rate overall, as confirmed by a significant main effect, F(1,25) = 10.1, p = 0.004, ηp2 = 0.29. Figure 3 suggests that nicotine and propranolol acted synergistically to alleviate the performance decrement over the course of the first block. This was confirmed by a significant nicotine x propranolol x period x block interaction, F(2,50) = 4.54, p = 0.015, ηp2 = 0.15. No other effects were significant in four-factor ANOVA.
Effects of nicotine and propranolol on hit rate in the Rapid Visual Information Processing Task. Averages are shown for each of three 5-minute time periods within two consecutive 15-min blocks. Blocks were separated by a short break. Error bars reflect SEMs, adjusted to remove between-subject variability in the average performance across drug conditions (Cousineau, 2007; Morey, 2008)
In separate three-factor ANOVAs of hit rate for each block, the nicotine x propranolol x time period interaction was significant for block 1, F(2,50) = 3.45, p = 0.039, ηp2 = 0.12, but not block 2, F(2,50) = 1.09, p = 0.34, ηp2 = 0.04. In block 1, a significant main effect of time period, reflecting performance decrement, was seen in all [Fs(2,50) > 10.6, ps < 0.001, ηp2 ≤ 0.30] except the Nicotine+Propranolol condition, F(2,50) = 1.93, p = 0.16, ηp2 = 0.07, confirming that both drugs combined alleviated the performance decrement with time on task. In block 2, there were significant main effects of nicotine, F(1,25) = 11.7, p = 0.002, ηp2 = 0.32, and propranolol, F(1,25) = 4.65, p = 0.041, ηp2 = 0.16, and effects appeared to be additive in the Nicotine+Propranolol condition. There were no significant interactions in the ANOVA of block 2. When comparing hit rate averaged over time periods within block 2 between the placebo and each of the three drug conditions, performance in the Nicotine+Propranolol session differed significantly from the Placebo session, t(25) = 3.92, p < 0.001, ηp2 = 0.38, but not performance in the Nicotine session, t(25) = 0.96, p = 0.35, ηp2 = 0.04, or Propranolol session, t(25) = .41, p = 0.69, ηp2 = 0.01.
RT
A significant main effect of time period, F(2,50) = 13.9, p < 0.001, ηp2 = 0.36, reflected RT slowing over time within each block. Nicotine shortened RT overall, F(1,25) = 14.3, p < 0.001, ηp2 = 0.36 (Figure 4). No other main effects or interactions were significant in four-factor ANOVA.
Effects of nicotine on reaction time in the Rapid Visual Information Processing Task. Propranolol had no significant effects. Measurements were averaged across time periods and blocks. Error bars reflect SEMs, adjusted to remove between-subject variability in the average performance across drug conditions (Cousineau, 2007; Morey, 2008). **p < 0.01, paired t-test
CDT
Significant main effects of set size on accuracy, F(1,25) = 296.5, p < 0.001, ηp2 = 0.92, and RT, F(1,25) = 120.6, p < 0.001, ηp2 = 0.83, reflected higher accuracy and shorter RT at set size 1 than at set size 5. Nicotine had no significant main effects or interactions. However, there were significant main effects of propranolol on accuracy, F(1,25) = 6.50, p = 0.017, ηp2 = 0.21, and RT, F(1,25) = 11.0, p = 0.003, ηp2 = 0.31. Figure 5 shows that propranolol increased accuracy and shortened RT across the presence and absence of nicotine. These effects did not interact with set size on either measure, Fs(1,25) ≤ 0.27, ps > 0.6, ηp2 ≤ 0.01.
Effects of propranolol in the Change Detection Task. Nicotine had no significant effects. Response accuracy and reaction time were averaged over set sizes. Error bars reflect SEMs, adjusted to remove between-subject variability in the average performance across drug conditions (Cousineau, 2007; Morey, 2008)
Discussion
The present study was designed to test the hypothesis, based on preclinical data, that propranolol would reverse cognitive-enhancing effects of nicotine, which would have provided evidence that nicotine-induced stimulation of noradrenaline release is involved in mediating these effects. In actual fact, propranolol helped uncover cognitive-enhancing effects of nicotine (or vice versa) in the RVIPT. Furthermore, propranolol enhanced performance in the CDT independent of the presence or absence of nicotine, and independent of whether one or five items had to be remembered suggesting that the effects were not mnemonic but attentional in nature. Propranolol also increased subjective self-reports of vigor. The only task showing no effects of propranolol was the SARAT, which was always performed first, probably before propranolol had reached peak plasma concentrations (Sharoky et al., 1988). Given that we did not use an extended-release formulation of propranolol, we had timed its administration such that Cmax would be reached on average 30 min after testing started. Thus, plasma levels may have been suboptimal at the time that the SARAT was performed.
The RVIPT is marked by intense speeded processing demands, which are difficult to uphold over time. β-adrenoceptor activation by nicotine-induced noradrenaline release might be expected to have stimulating effects that would help sustain attention under these conditions. Reports that stimulants help sustain attention (reviewed by Edgar, Pace-Schott, & Wesnes, 2009; Koelega, 1993) have reinforced the view that heightened arousal, as by direct or indirect noradrenergic stimulation, is beneficial to performance of such tasks (Espana, Schmeichel, & Berridge, 2016). However, the present additive and even synergistic beneficial effects of nicotine and propranolol in the RVIPT suggest that downstream β-adrenoceptor activation limited the performance-enhancing effects of nicotine, as they were unmasked by β-adrenoceptor antagonism. It is conceivable that RVIPT performance itself created elevated arousal, and that under these conditions additional β-adrenoceptor stimulation following nicotine administration was detrimental. Several studies found that propranolol can benefit performance in tasks of cognitive flexibility (reviewed by Beversdorf, 2019) and alleviate the negative impact of stress-related arousal thereon (Alexander, Hillier, Smith, Tivarus, & Beversdorf, 2007). Furthermore, propranolol attenuated beneficial effects of elevated arousal on long-term memory (Cahill, Prins, Weber, & McGaugh, 1994; Hauser, Eldar, Purg, Moutoussis, & Dolan, 2019) and had negative effects on working memory only against a background of low emotional arousal (Muller, Mottweiler, & Bublak, 2005). Thus, many of the performance-modulating effects of propranolol could be summarized as effects of lowering arousal, which can be beneficial for specific task demands or against a baseline of hyperarousal.
However, other effects of propranolol in the present study are difficult to explain in this manner. Propranolol benefited performance of the unspeeded CDT, a task that is not likely to have promoted elevated arousal. The effects of propranolol on the vigor scale of the POMS, which was administered before and after but not during cognitive testing, are also consistent with an overall beneficial effects profile on attentiveness and not with specificity of effects for conditions of high testing-induced arousal. Thus, just as a unidimensional arousal construct has not held up to empirical evidence (Robbins & Everitt, 1996), the present findings suggest that an interpretation of propranolol effects in terms of lowered arousal would be oversimplified.
The interpretation of the present results may benefit from considering differences to the previous study employing the rat 5-CSRTT (Hahn & Stolerman, 2005), in which propranolol blocked the attention-enhancing effects of nicotine and tended to impair performance when given alone. That study found impairment with propranolol only with a fast stimulus presentation rate, and not with a longer intertrial interval which required periods of watchful inactivity. The detrimental performance effects of propranolol with the fast presentation rate cannot be explained by response rate-depressant effects, because they were seen on a rate-independent measure of response choice. However, the faster presentation rate also creates a greater requirement for physical reorienting in space because reward after each 5-CSRTT trial is delivered in the wall opposite the target locations. In contrast, the present study created sustained processing demands while staying physically stationary for prolonged periods of time without any spatial orienting requirements. The preceding 5-hour absorption period, also spent in physical inactivity, may have made this particularly challenging, as supported by decreases in self-reported vigor and increases in tension over this time period. Propranolol reversed the increase in tension over the course of the absorption period. Thus, lowering β-adrenergic tone may increase tolerance for prolonged periods of forced physical inactivity and promote the ability to stay focused under these conditions.
The interpretation of the present results may be furthered by considering different attention and arousal functions associated with different neurotransmitter systems (Robbins & Everitt, 1996). The functions ascribed to the acetylcholine (ACh) system were summarized as “sustained attention and mental endurance […] in monitoring well-learned actions, suppression of these actions and/or waiting for special but expected events to occur in a tonic state regulated by a parasympathetic (also ACh-supervised) system” (Trofimova & Robbins, 2016). These functions appear to play a large role for task performance under the conditions of the present study. In contrast, the functions upheld by the noradrenergic system are most commonly described as regulating orientation and attention to novelty, especially under conditions of uncertainty and exploration (Trofimova & Robbins, 2016). These functions were clearly not essential to performance in the present study, consistent with a lack of impairment by β-adrenoceptor antagonism. While no specific attention and arousal functions were ascribed to β-subtypes of adrenoceptors specifically, their association with the sympathetic nervous system would be consistent with a role in opposing endurance-related functions of the ACh-supervised parasympathetic nervous system. In the present study, benefits of nicotine-induced ACh release may have been counteracted by nicotine-induced noradrenaline release and uncovered by β-adrenoceptor blockade. In contrast, performance under conditions requiring reorienting to unpredictable target locations, as in the rat 5-CSRTT, is likely to depend on orienting functions upheld by the noradrenergic system more than on sustained attention functions upheld by the ACh system. This would be consistent with findings that 5-CSRTT performance is not sensitive to task manipulations that should alter sustained attention demands (Hahn, Shoaib, & Stolerman, 2002b), but is sensitive to β-adrenoceptor tone (Hahn & Stolerman, 2005).
A limitation of the present study is a moderate sample size, which precluded examination of individual differences and potential moderators, such as sex or personality traits. Another limitation is the use of a single dose of both nicotine and propranolol. The study’s factorial design would have necessitated adding at least two test sessions for each additional dose level of either drug, which would have made participant retention challenging. Furthermore, the attention-enhancing effects of nicotine were unusually weak compared to previous studies employing the same paradigms in smokers and non-smokers (e.g., Hahn et al., 2007; Hahn et al., 2020). Side effects were carefully monitored and are very unlikely to have interfered with the measurement of cognitive benefits. Whatever the reason, the weak effects of nicotine made it difficult to address the question of whether propranolol may attenuate them. Finally, we did not verify propranolol blood levels. Given that one participant (whose data were excluded) later reported secretly spitting out the capsule, this appears a nontrivial omission. However, all except four participants showed a clear reduction in heart rate in the sessions involving propranolol administration relative to sessions without propranolol. The four subjects who did not show a drop in heart rate displayed a robust decrease in blood pressure in the propranolol sessions. Thus, we are confident that propranolol was absorbed in all participants.
In summary, cognitive effects of changes in β-adrenoceptor tone appear to depend on task context. Critical factors may include the need for stimulus-induced orienting under conditions of uncertainty versus mental endurance under conditions of well-practiced, predictable processing demands. Furthermore, our results suggest that the downstream mechanisms responsible for the cognitive-enhancing effects of nAChR agonists depend on context in a similar manner. Finally, the present results raise the interesting possibility that moderate doses of propranolol may be useful for enhancing sustained information processing ability under conditions of prolonged forced physical inactivity, such as frequently encountered in academic settings.
References
Adams, C. E., & Stevens, K. E. (2007). Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Frontiers in Bioscience, 12, 4755-4772.
Alexander, J. K., Hillier, A., Smith, R. M., Tivarus, M. E., & Beversdorf, D. Q. (2007). Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience, 19(3), 468-478. https://doi.org/10.1162/jocn.2007.19.3.468
Aston-Jones, G., Chiang, C., & Alexinsky, T. (1991). Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research, 88, 501-520. https://doi.org/10.1016/s0079-6123(08)63830-3
Aston-Jones, G., & Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403-450. https://doi.org/10.1146/annurev.neuro.28.061604.135709
Aston-Jones, G., Rajkowski, J., Kubiak, P., & Alexinsky, T. (1994). Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. The Journal of Neuroscience, 14(7), 4467-4480.
Beversdorf, D. Q. (2019). Neuropsychopharmacological regulation of performance on creativity-related tasks. Current Opinion in Behavioral Sciences, 27, 55-63. https://doi.org/10.1016/j.cobeha.2018.09.010
Cahill, L., Prins, B., Weber, M., & McGaugh, J. L. (1994). Beta-adrenergic activation and memory for emotional events. Nature, 371(6499), 702-704. https://doi.org/10.1038/371702a0
Cohen, J. (1988). Statistical power analysis for the behavioral sciences, 2nd Erlbaum, Hillsdale, NJ.
Cousineau, D. (2007). Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorial in Quantitative Methods for Psychology, 1, 42-45.
Edgar, C. J., Pace-Schott, E. F., & Wesnes, K. A. (2009). Approaches to measuring the effects of wake-promoting drugs: a focus on cognitive function. Human Psychopharmacology, 24(5), 371-389. https://doi.org/10.1002/hup.1034
Espana, R. A., Schmeichel, B. E., & Berridge, C. W. (2016). Norepinephrine at the nexus of arousal, motivation and relapse. Brain Research, 1641(Pt B), 207-216. https://doi.org/10.1016/j.brainres.2016.01.002
Fant, R. V., Henningfield, J. E., Shiffman, S., Strahs, K. R., & Reitberg, D. P. (2000). A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacology, Biochemistry, and Behavior, 67(3), 479-482.
Foulds, J., Stapleton, J., Swettenham, J., Bell, N., McSorley, K., & Russell, M. A. (1996). Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology, 127(1), 31-38.
Gorsline, J., Gupta, S. K., Dye, D., & Rolf, C. N. (1993). Steady-state pharmacokinetics and dose relationship of nicotine delivered from Nicoderm (Nicotine Transdermal System). Journal of Clinical Pharmacology, 33(2), 161-168.
Grier, J. B. (1971). Nonparametric indexes for sensitivity and bias: computing formulas. Psychological Bulletin, 75(6), 424-429.
Gupta, S. K., Benowitz, N. L., Jacob, P., 3rd, Rolf, C. N., & Gorsline, J. (1993). Bioavailability and absorption kinetics of nicotine following application of a transdermal system. British Journal of Clinical Pharmacology, 36(3), 221-227.
Hahn, B. (2015). Nicotinic receptors and attention. Current Topics in Behavioral Neurosciences, 23, 103-135. https://doi.org/10.1007/978-3-319-13665-3_5
Hahn, B., Harvey, A. N., Concheiro-Guisan, M., Huestis, M. A., Holcomb, H. H., & Gold, J. M. (2013). A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biological Psychiatry, in press
Hahn, B., Ross, T. J., & Stein, E. A. (2006). Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage, 32(2), 842-853. https://doi.org/10.1016/j.neuroimage.2006.04.177
Hahn, B., Ross, T. J., Yang, Y., Kim, I., Huestis, M. A., & Stein, E. A. (2007). Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. The Journal of Neuroscience, 27(13), 3477-3489.
Hahn, B., Shoaib, M., & Stolerman, I. P. (2002a). Effects of dopamine receptor antagonists on nicotine-induced attentional enhancement. Behavioural Pharmacology, 13(8), 621-632.
Hahn, B., Shoaib, M., & Stolerman, I. P. (2002b). Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task-demands. Psychopharmacology, 162(2), 129-137.
Hahn, B., Shrieves, M. E., Olmstead, C. K., Yuille, M. B., Chiappelli, J. J., Pereira, E. F. R., … Fawcett, W. P. (2020). Evidence for positive allosteric modulation of cognitive-enhancing effects of nicotine in healthy human subjects. Psychopharmacology, 237(1), 219-230. https://doi.org/10.1007/s00213-019-05363-4
Hahn, B., & Stolerman, I. P. (2005). Modulation of nicotine-induced attentional enhancement in rats by adrenoceptor antagonists. Psychopharmacology, 177(4), 438-447.
Hauser, T. U., Eldar, E., Purg, N., Moutoussis, M., & Dolan, R. J. (2019). Distinct roles of dopamine and noradrenaline in incidental memory. The Journal of Neuroscience https://doi.org/10.1523/JNEUROSCI.0401-19.2019
Haydar, S. N., & Dunlop, J. (2010). Neuronal nicotinic acetylcholine receptors - targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer's disease. Current Topics in Medicinal Chemistry, 10(2), 144-152.
Heishman, S. J., Kleykamp, B. A., & Singleton, E. G. (2010). Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology, 210(4), 453-469. https://doi.org/10.1007/s00213-010-1848-1
Hong, L. E., Yang, X., Wonodi, I., Hodgkinson, C. A., Goldman, D., Stine, O. C., … Thaker, G. K. (2011). A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes, Brain, and Behavior, 10(5), 530-535. https://doi.org/10.1111/j.1601-183X.2011.00689.x
Hurst, R., Rollema, H., & Bertrand, D. (2013). Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacology & Therapeutics, 137(1), 22-54. https://doi.org/10.1016/j.pharmthera.2012.08.012
Kendziorra, K., Wolf, H., Meyer, P. M., Barthel, H., Hesse, S., Becker, G. A., … Sabri, O. (2011). Decreased cerebral alpha4beta2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer's disease assessed with positron emission tomography. European Journal of Nuclear Medicine and Molecular Imaging, 38(3), 515-525. https://doi.org/10.1007/s00259-010-1644-5
Koelega, H. S. (1993). Stimulant drugs and vigilance performance: a review. Psychopharmacology, 111(1), 1-16.
Lopez, E., Arce, C., Vicente, S., Oset-Gasque, M. J., & Gonzalez, M. P. (2001). Nicotinic receptors mediate the release of amino acid neurotransmitters in cultured cortical neurons. Cerebral Cortex, 11(2), 158-163.
Luck, S. J., & Vogel, E. K. (1997). The capacity of visual working memory for features and conjunctions. Nature, 390(6657), 279-281. https://doi.org/10.1038/36846
MacDermott, A. B., Role, L. W., & Siegelbaum, S. A. (1999). Presynaptic ionotropic receptors and the control of transmitter release. Annual Review of Neuroscience, 22, 443-485.
McNair, D. M., Lorr, M., & Droppleman, L. F. (1971). Profile of mood states manual. San Diega, CA: Educational and Industrial Testing Service.
Morey, R. D. (2008). Confidence intervals from normalized data: a correction of Cousineau. Tutorial in Quantitative Methods for Psychology, 4, 61-64.
Muller, U., Mottweiler, E., & Bublak, P. (2005). Noradrenergic blockade and numeric working memory in humans. Journal of Psychopharmacology, 19(1), 21-28. https://doi.org/10.1177/0269881105048888
Newhouse, P. A., Potter, A. S., Dumas, J. A., & Thiel, C. M. (2011). Functional brain imaging of nicotinic effects on higher cognitive processes. Biochemical Pharmacology, 82(8), 943-951. https://doi.org/10.1016/j.bcp.2011.06.008
Perry, E., Martin-Ruiz, C., Lee, M., Griffiths, M., Johnson, M., Piggott, M., …. Court, J. (2000). Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. European Journal of Pharmacology, 393(1-3), 215-222.
Petrovsky, N., Quednow, B. B., Ettinger, U., Schmechtig, A., Mossner, R., Collier, D. A., … Kumari, V. (2010). Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology, 35(7), 1429-1439. https://doi.org/10.1038/npp.2010.12
Quarta, D., Naylor, C. G., Glennon, J. C., & Stolerman, I. P. (2012). Serotonin antagonists in the five-choice serial reaction time task and their interactions with nicotine. Behavioural Pharmacology, 23(2), 143-152.
Quarta, D., Naylor, C. G., Morris, H. V., Patel, S., Genn, R. F., & Stolerman, I. P. (2007). Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology, 53(3), 421-430.
Radek, R. J., Kohlhaas, K. L., Rueter, L. E., & Mohler, E. G. (2010). Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Current Pharmaceutical Design, 16(3), 309-322.
Rezvani, A. H., Caldwell, D. P., & Levin, E. D. (2005). Nicotinic-serotonergic drug interactions and attentional performance in rats. Psychopharmacology, 179(3), 521-528.
Robbins, T. W., & Everitt, B. J. (1996). Arousal Systems and Attention. In M. Gazzaniga (Ed.), The Cognitive Neurosciences (pp. 703-720). Cambridge, MA: MIT Press.
Role, L. W., & Berg, D. K. (1996). Nicotinic receptors in the development and modulation of CNS synapses. Neuron, 16(6), 1077-1085.
Rollema, H., Hajos, M., Seymour, P. A., Kozak, R., Majchrzak, M. J., Guanowsky, V., … Williams, K. E. (2009). Preclinical pharmacology of the alpha 4 beta 2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochemical Pharmacology, 78(7), 813-824.
Sharoky, M., Perkal, M., Turner, R., & Lesko, L. J. (1988). Steady state relative bioavailability and pharmacokinetics of oral propranolol in black and white North Americans. Biopharmaceutics & Drug Disposition, 9(5), 447-456.
Trofimova, I., & Robbins, T. W. (2016). Temperament and arousal systems: A new synthesis of differential psychology and functional neurochemistry. Neuroscience and Biobehavioral Reviews, 64, 382-402. https://doi.org/10.1016/j.neubiorev.2016.03.008
Usher, M., Cohen, J. D., Servan-Schreiber, D., Rajkowski, J., & Aston-Jones, G. (1999). The role of locus coeruleus in the regulation of cognitive performance. Science, 283(5401), 549-554. https://doi.org/10.1126/science.283.5401.549
Warburton, D. M., & Mancuso, G. (1998). Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology, 135(3), 305-310.
Wesnes, K., & Warburton, D. M. (1984). Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology, 82(3), 147-150. https://doi.org/10.1007/bf00427761
Wing, V. C., Wass, C. E., Soh, D. W., & George, T. P. (2012). A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Annals of the New York Academy of Sciences, 1248, 89-106. https://doi.org/10.1111/j.1749-6632.2011.06261.x
Wonnacott, S., Barik, J., Dickinson, J., & Jones, I. W. (2006). Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. Journal of Molecular Neuroscience, 30(1-2), 137-140.
Yuille, M. B., Olmstead, C. K., Wells, A. K., & Hahn, B. (2017). A test of the cognitive-enhancing potential of low-dose mecamylamine in healthy non-smokers. Psychopharmacology, 234(1), 109-116. https://doi.org/10.1007/s00213-016-4443-2
Acknowledgements
This work was funded by National Institutes of Health grant R01 DA035813 to B. Hahn. We would like to thank Dr. Ian Stolerman for commenting on a draft of this manuscript.
Open Practices Statement
None of the data or materials for the experiments reported here is available, and none of the experiments was preregistered.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was funded by the National Institutes of Health (R01 DA035813 to B. Hahn). The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Hahn, B., Olmstead, C.K., Yuille, M.B. et al. Attention-enhancing effects of propranolol and synergistic effects with nicotine. Cogn Affect Behav Neurosci 20, 658–668 (2020). https://doi.org/10.3758/s13415-020-00794-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-020-00794-5