Abstract
Social learning theory postulates that individuals learn to engage in aggressive behavior through observing an aggressive social model. Prior studies have shown that repeatedly observing aggression, also called “chronic passive exposure to aggression,” changes accumbal dopamine D2 receptor (D2R) and amygdaloid 5-HT1B receptor (5-HT1BR) densities in observers. But, the association between these outcomes remains unknown. Thus, in our study, we used a rat paradigm to comprehensively examine the linkage between aggression, D2R density in the nucleus accumbens core (AcbC) and shell (AcbSh), and 5-HT1BR density in the medial (MeA), basomedial (BMA), and basolateral (BLA) amygdala following chronic passive exposure to aggression. Male Sprague-Dawley rats (N = 72) were passively exposed to either aggression or nonaggression acutely (1 day) or chronically (23 days). When observer rats were exposed to aggression chronically, they showed increased aggressive behavior and reduced D2R density in bilateral AcbSh. On the other hand, exposure to aggression, regardless of exposure length, increased the 5-HT1BR density in bilateral BLA. Finally, low D2R in the AcbSh significantly interacted with high 5-HT1BR density in the BLA to predict high levels of aggression in observer rats. Our results advance our understanding of the neurobiological mechanisms in the observational learning of aggression, highlighting that dopamine–serotonin interaction, or AcbSh–BLA interaction, may contribute to a risk factor for aggression in observers who chronically witness aggressive interactions.
Similar content being viewed by others
According to social learning theory (Bandura, 1973, 1977; Bandura, Ross, & Ross, 1961, 1963), youths are inclined to engage in aggressive behavior after they have observed an aggressive adult model. Notably, this theory provides a psychosocial explanation for aggression in bystanders, who are not actually involved in violent situations. Long-lasting events of observing violence may particularly cause observer youths to adopt aggressive behavior (Huesmann & Kirwil, 2007). In fact, previous literature has shown evidence consistent with this social learning theory. For example, individuals who have witnessed community and family violence in childhood tend to show aggressive and other externalizing behaviors (Guerra, Huesmann, Tolan, Van Acker, & Eron, 1995; Holmes, 2013), child abuse (Widom, 1989), positive attitudes toward aggression (Guerra, Huesmann, & Spindler, 2003; Su, Mrug, & Windle, 2010), and aggressive fantasies (Su et al., 2010). These behavioral effects of witnessing violence, also known as “passive exposure to aggression,” have been found in animal studies as well; fish and rodents show aggressive tendencies following repeatedly observing fights between conspecifics (Clotfelter & Paolino, 2003; Feldker et al., 2006; Suzuki & Lucas, 2010; Welch & Welch, 1971). Therefore, it is reasonable to suggest that chronic passive exposure to aggression is a risk factor for the observers’ aggressiveness.
Yet, given that there are various forms of aggression, it is important to clarify what type of aggression particularly increases among observers who have been chronically exposed to aggressive situations. Traditionally, aggression is classified into two types: impulsive (or hostile/reactive) aggression, which is primarily driven by negative emotional states, and instrumental (or premeditated/proactive) aggression, which is a type of hurting behavior aiming to achieve some other end (Anderson & Huesmann, 2003; Nelson & Trainor, 2007; Vitiello & Stoff, 1997). Because previous research has shown that chronic passive exposure to aggression is broadly associated with impulsive, risk-taking behavior (Margolin & Gordis, 2000), including not only aggression (as discussed earlier) but also externalizing problems (Bauer et al., 2006; Emery, 2011; Fantuzzo et al., 1991) and illegal drug use (Berenson, Wiemann, & McCombs, 2001; Kilpatrick et al., 2000; Sussman, Dent, & McCullar, 2000; Sussman, Dent, & Stacy, 1999; Vermeiren, Schwab-Stone, Deboutte, Leckman, & Ruchkin, 2003), chronic exposure to aggression is conceivably associated with impulsive aggression. Furthermore, prior findings have indicated that chronic passive exposure to aggression increases aggressive behavior solely, without altering defensive/submissive behavior (Suzuki & Lucas, 2010). This possibly suggests that observers may increase their fearless aggression (as opposed to rage- or fear-induced aggression)—that is, the risk-seeking properties of impulsive aggression.
Indeed, the possible association between chronic passive exposure to aggression and risk-seeking/impulsive aggression has been implied by previous neurochemical studies. For instance, rats exposed to aggression for 23 consecutive days show downregulated dopamine D2 receptor (D2R) density in the shell of the nucleus accumbens (AcbSh) bilaterally, as compared to those exposed to nonaggression for the same number of days (Suzuki, Han, & Lucas, 2010a). In general, the accumbal dopaminergic system has been implicated in motivation for hedonic rewards (Berridge, 2007), and dopamine release in the AcbSh is stimulated following risk-seeking/impulsive behaviors, such as alcohol consumption (Bustamante et al., 2008; van Erp & Miczek, 2007) and psychostimulant drugs (Desai, Paronis, Martin, Desai, & Bergman, 2010; Kleijn et al., 2012). Interestingly, dopamine release in the nucleus accumbens is similarly triggered following aggression (Beiderbeck et al., 2012; Ferrari, van Erp, Tornatzky, & Miczek, 2003; van Erp & Miczek, 2000), suggesting that aggression may serve as impulsively fulfilling demands for dopamine reward outputs. Moreover, a D2R antagonist (sulpiride or haloperidol) infused into the nucleus accumbens decreased aggressive behavior (Beiderbeck et al., 2012; Couppis & Kennedy, 2008), although this pharmacological manipulation broadly influenced both the core of the nucleus accumbens (AcbC) and AcbSh. Thus, the accumbal dopaminergic system may be related to the rewarding properties of aggression (Couppis & Kennedy, 2008).
Observer rats exposed to aggression for 23 days also show upregulated serotonin 5-HT1B receptor (5-HT1BR) density in the basolateral amygdala (BLA), as compared to controls (Suzuki, Han, & Lucas, 2010b). The serotonergic system generally functions to regulate aggression; low 5-HT levels are often associated with aggressive traits (Caramaschi, de Boer, de Vries, & Koolhaas, 2008; Ferris et al., 1997; Ferris, Stolberg, & Delville, 1999; Pihl & Benkelfat, 2005). Among brain regions, the amygdala shows a high concentration of 5-HT, 5-HIAA (indicating 5-HT synthesis), and serotonin transporter in neurons, as compared to the prefrontal cortex or hippocampus (Arrant, Jemal, & Kuhn, 2013). Aggressive motivation increases functional activation in the amygdala, including the medial (MeA), basomedial (BMA), and BLA, and this aggression-related amygdala activity is suppressed by a selective serotonin reuptake inhibitor (fluoxetine; Ferris et al., 2008). This may indicate the involvement of the amygdaloid 5-HT system in aggression. Furthermore, a high number of 5-HT1BR-positive neurons in the BLA may be associated with impulsive, “pathological” aggression (Jacobs, Van Den Broeck, & Simoens, 2007), whereas pharmacologically induced deletion of serotonergic fibers in the BLA increases fear-potentiated startle (Tran, Lasher, Young, & Keele, 2013). These findings suggest that the serotonergic system in the amygdala, especially in the BLA, may be critical in the “fight-or-flight” response to a potentially threatening situation (Cannon, 1939). That is, a “fight” response may tend to be activated more often than a “flight” response, depending on an individual’s social experience, stress vulnerability, and 5-HT activity (D. C. Blanchard & Blanchard, 1990; D. C. Blanchard, Sakai, McEwen, Weiss, & Blanchard, 1993; D. C. Blanchard et al., 1995; R. J. Blanchard, Yudko, Dulloog, & Blanchard, 2001; Koolhaas, de Boer, Buwalda, & van Reenen, 2007; Koolhaas et al., 1999; Koolhaas, Meerlo, De Boer, Strubbe, & Bohus, 1997; Tamashiro, Nguyen, & Sakai, 2005).
Together, the accumbal dopaminergic activity and the amygdaloid serotonergic activity appear to be involved in impulsive aggressive behavior. This suggests the possibility that the alterations in D2R density in the AcbSh and 5-HT1BR density in the BLA following chronic passive exposure to aggression, as shown in prior studies (Suzuki et al., 2010a, 2010b), might contribute to impulsive aggressive behavior in observers (Clotfelter & Paolino, 2003; Feldker et al., 2006; Suzuki & Lucas, 2010; Welch & Welch, 1971). However, to our knowledge, no studies have directly examined an interplay between these local receptor densities and aggressive behavior following chronic passive exposure to aggression. Furthermore, no studies have directly compared these local receptor densities in acute versus chronic passive exposure to aggression.
To clarify the two questions above, the present study was designed to follow up on prior studies examining the effects of passive exposure to aggression. Specifically, the present study was conducted to quantify impulsive aggression, D2R density in the nucleus accumbens (AcbC and AcbSh), and 5-HT1BR density in the amygdala (MeA, BMA, and BLA) within observer rats and compare them between acute and chronic passive exposure to aggression. To achieve this goal, we developed a rat paradigm specifically tailored to test our hypothesis of observer-learned aggression (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). Notably, it was important to contrast acute exposure with chronic exposure in order to illustrate whether the observer rats increased their aggressiveness due to “mimicry/priming” effects or to “observational learning” effects (Huesmann & Kirwil, 2007; Suzuki & Lucas, 2010). That is, if chronic exposure to aggression resulted in more aggression in observer rats than did acute exposure to aggression, this would likely indicate that observers’ aggression was induced by a long-term observational learning process, rather than by just an instant imitation of aggression (which would then be seen immediately after an acute exposure). Therefore, in this paradigm, we administered acute or chronic exposure session(s) right before a behavioral assessment of aggression for an observer rat (see Fig. 1), which was suitable for our purpose.
In the present study, we aimed to test three hypotheses. The first hypothesis was that chronic passive exposure to aggression would not only result in increased impulsive aggressive behavior, as had been reported previously (Suzuki & Lucas, 2010), but also in downregulated D2R density in the AcbSh and upregulated 5-HT1BR density in observer rats, as compared to acute exposure to aggression. The second hypothesis was that the changes in the identified local receptor densities, especially D2R density in the AcbSh and 5-HT1BR density in the BLA, would be associated with each other. The third hypothesis was that increased impulsive aggression would be associated with the identified local receptor densities, especially D2R density in the AcbSh and/or 5-HT1BR density in the BLA.
Method
Subjects
Seventy-two young male Sprague-Dawley rats were bred in our Animal Care Facilities (ACF) and reared in a group (cage size = 47 × 25.5 × 21.5 cm). When they weighed 150–250 g, they were individually housed and equally assigned to one of four conditions (n = 18 each): (1) acute exposure to nonaggression (AN), (2) chronic exposure to nonaggression (CN), (3) acute exposure to aggression (AA), or (4) chronic exposure to aggression (CA). The purpose of having AN and AA rats was to examine the mimicry/priming effects of passive exposure to aggression, whereas the purpose of having CN and CA rats was to examine the observational learning effects of aggression (see Fig. 1). This between-group design signified whether repeated exposure, while ruling out a possible priming effect immediately following exposure, was required for observer rats to behave aggressively.
The total sample size was determined by a prospective power analysis of our pilot behavioral data in the past (Suzuki & Lucas, 2010). On the basis of a 2 (exposure length: acute vs. chronic) × 2 (exposure condition: exposure to nonaggression vs. exposure to aggression) analysis of variance (ANOVA), the estimated values of Cohen’s d were the following: 0.4 for the main effect of exposure length, 0.6 for the main effect of exposure condition, and 0.95 for the interaction between them. A power analysis indicated that the total sample size of 72 would attain 95% power to detect the effect of exposure length, 97% power to detect the effect of exposure condition, and 100% power to detect the interaction effect. Therefore, the present study assured adequate power.
All observer rats were given ad libitum (oval pellet-typed food for laboratory rodents, LabDiet 5001 Rodent Diet, Southern Agriculture, Tulsa, OK) and water in a climatized room (temperature = 21–22 °C; humidity = 30%–60%; 12-h light:dark cycle; lights on at 7:00 a.m., lights off at 7:00 p.m.) under the approval of the Loyola University Chicago Institutional Animal Care and Use Committee (IACUC).
Additional rats for inducing aggressive contexts
Additional male Sprague-Dawley rats were inbred in our ACF and prepared to manipulate the aggressive or nonaggressive control contexts that observer rats were exposed to. First, behavioral screening tests were administered to select the six most nonaggressive rats and the six most aggressive rats (body weights ≥ 400 g). Next, starting from 2 weeks prior to an experiment, each nonaggressive rat was housed with a younger male rat (body weight = 100 g less than the nonaggressive rat), whereas each aggressive rat was housed with a female rat (body weight = 250 g). This 2-week cohabitation (1) allowed the nonaggressive male–male dyad to establish a social hierarchy or (2) provoked aggressive motivation among the aggressive male rats having a female partner (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010).
During the experiment, a nonaggressive dyad was presented to the AN and CN groups. The nonaggressive dyad was less likely to show aggression because they were motivated to maintain a social hierarchy and did not need to fight for sorting out their rank. In contrast, the aggressive male rat was separated from a female partner; paired with a younger naïve male rat (body weight = 100 g less than the aggressive rats); and then presented to the AA and CA groups. Because this naïve male rat was a potential rival for mating and territory, the aggressive rat was likely to show intermale and territorial aggression (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). In this way, this male–male pair served as an aggressive dyad. After the experiment, the aggressive rat was separated from the naïve rat and paired with the female partner again. All nonaggressive and aggressive dyads were repeatedly used until they no longer behaved their expected roles. The Loyola University Chicago IACUC approved the use of nonaggressive dyads, aggressive dyads, female partners, and young male rats (the approximate number of rats = 156 rats) during our experiment.
Procedure
The procedure was identical to a previously established protocol (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). Under a red-light illumination between 7:00 and 9:00 p.m., each observer rat was transferred from his home cage to a small plastic and transparent aquarium with a mesh lid (cage size = 22.9 × 15 × 16.5 cm). Note that this aquarium had enough space for a rat to move around freely; thus, potential restraint stress was minimal. Then, the observer rat in the aquarium was placed into the cage (47 × 25.5 × 21.5 cm) of either the nonaggressive dyad (for the AN and CN groups) or the aggressive dyad (for the AA and CA groups). Importantly, the observer rats could not make any physical contact with the nonaggressive/aggressive dyad, but they could see, hear, and smell the dyad through the mesh lid or transparent barrier. This observational session took 10 min per day and was recorded by a video camera. Immediately after the session, the observer rat was removed from the aquarium and placed back in his home cage.
The observational session was conducted only one time (for the AN and AA groups) or was repeated once daily for 23 consecutive days (for the CN and CA groups). Additionally, the CN and CA rats were cycled to pair a different dyad each day, minimizing within-group variability in the amounts of observing nonaggression or aggression.
As soon as the last observational session was done, a 10-min behavioral screening test was conducted under red-light illumination (between 7:10 and 9:30 p.m.) to assess the aggressiveness of each observer rat. In this screening test, each observer rat was paired with another naïve male rat, called an opponent rat, in a new cage (cage size = 47 × 25.5 × 21.5 cm), and their social interactions were recorded by a video camera. The opponent rat was weight-matched to the observer rat so that it was physically fair for both rats during a fight. Given such a nonhandicapped fight, if the observer rat maintained aggressive behavior for a long time (regardless of whether the opponent rat attacked/counterattacked or even became dominant in several fights), this was operationally defined as impulsive aggression. Immediately after the final screening test, the observer rats were decapitated to collect blood and brain samples. Blood samples were centrifuged at 2,500 rpm at 4 °C for 15 min to extract serum, which was stored at −20 °C until it was used. Brain samples were removed rapidly, frozen on powdered dry ice, and stored at −70 °C until used.
Note that the behavioral screening test and the following decapitation were performed as soon as the last observational session was completed, which modified a previous protocol (Suzuki et al., 2010a, 2010b; Suzuki & Lucas, 2010). This was done primarily because it was necessary to check stress hormone corticosterone levels following exposure before hormone levels returned to baseline. Given that stress could potentially induce aggression (Wood, Norris, Waters, Stoldt, & McEwen, 2008; Wood, Young, Reagan, & McEwen, 2003; Yohe, Suzuki, & Lucas, 2012), the present protocol was helpful in confirming whether acute and chronic exposure to aggression did not produce unexpected stress. Furthermore, the present protocol helped us clarify whether any change in the target receptor density occurred slowly (e.g., in the 24 h following the exposure session; see Suzuki et al., 2010a, 2010b), or rapidly (e.g., even immediately following the exposure session). For these reasons, decapitation was performed as soon as the exposure session and behavioral testing were conducted.
Aggression assessment
Trained raters counted up the amount of time (in seconds) during which the observer rats, as well as the opponent rats, were engaged in aggressive behavior, using a stopwatch. Aggression from the opponent rats was used as background information.
Aggressive behavior was measured according to a previously published protocol (Miczek, 1974; Suzuki & Lucas, 2010). Specifically, the following actions were considered aggressive behavior: attack (e.g., leaping at an opponent, pulling an opponent’s skin), threat (e.g., pushing an opponent with his back), aggressive posture (e.g., bending over an opponent with his head and forelimbs arched over an opponent), allogrooming (e.g., aggressively grooming or nibbling an opponent’s neck), mutual upright posture (e.g., standing on his hindlegs and boxing), and chasing (e.g., following an fleeing opponent). Play fighting (e.g., contacting each other’s snout, face, and nape of the neck) was excluded (Pellis & Pellis, 1987; Pellis, Pellis, & Foroud, 2005) when both the observer rats and the opponent rats were assessed. Interrater reliability of all behavioral scores met the acceptable level (Kline, 1999): The Cronbach’s α values were .84 for the aggression of the observer rats and .79 for the aggression of the opponent rats.
Radioimmunoassay
To check background information, levels of serum testosterone and corticosterone were assayed using the commercially available radioimmunoassay kits Coat-A-Count Total Testosterone and Coat-A-Count Rat Corticosterone (Siemens, Los Angeles, CA). Following the protocols in the kits, the concentrations of serum testosterone and corticosterone were computed from a logit–log calibration curve, which was drawn from radioactive counts and concentrations of the calibrators.
Brain sectioning and receptor binding autoradiography
Coronal sections of 20-μm thickness were cut on a cryostat at –15 °C and thaw-mounted onto 12 glass microscope slides (Superfrost Plus, VWR West Chester PA; four sections per slide). The target sections included the accumbal areas (i.e., AcbC and AcbSh, between 2.52 and 1.56 mm prior to bregma) and the amygdaloid areas (i.e., MeA, BMA, and BLA, between 2.16 and 3.12 mm posterior to bregma), identified according to the atlas by Paxinos and Watson (2005). All brain sections were stored at –70 °C until used.
At the time of chemical processing, the two best, cross-matched slides were selected from each group of observer rats. Slides containing the accumbal sections were processed for D2R binding autoradiography in the following way: (1) rinsing them twice with 50 mM of Tris HCl (pH 7.4) for 10 min, (2) incubating them in a buffer solution [containing 50 mM of Tris HCl (pH 7.4), 120 mM of NaCl, 5 mM of KCl, 2 mM of CaCl2, 1 mM of MgCl2, 100 pM of [125I]2'-iodospiperone, and 50 nM of ketanserin] at room temperature for 90 min, (3) rinsing them in a cold 50 mM of Tris HCl (pH 7.4) three times for 10 min per wash, (4) dipping them quickly in ice-cold double-distilled H2O for less than 5 s, (5) drying them under a stream of cool air, (6) placing them in cassettes and exposing them, in addition to 125I plastic standards (ranging from 11.5 to 6000 μCi/g; American Radiolabeled Chemicals, Inc., St. Louis, MO), to BioMax MR film (Kodak), and (7) leaving them under a dark area for 8 h. For nonspecific binding, one additional slide from each group was processed in the same way described above, except that 100 μM of SCH23390 was added to the buffer solution.
Slides containing the amygdaloid sections were processed for 5-HT1BR binding autoradiography in the following way: (1) incubating them in a buffer solution [containing 170 mM of Tris HCl (pH 7.4), 150 mM of NaCl, 50 pM of [125I]cyanopindolol, 100 nM of 8-OH-DPAT, and 30 μM of isoproterenol] at room temperature for 120 min, (2) rinsing them in cold binding buffer solution two times for 5 min per wash, (3) dipping them quickly in ice-cold double-distilled H2O at 4 °C for less than 5 s, (4) drying them under a stream of cool air, (5) placing them in cassettes and exposing them and 125I plastic standards to BioMax MR film (Kodak), and (6) leaving them under a dark area for 88 h. For nonspecific binding, one more slide was selected from each group and processed in the same way described above, except that 100 μM of raclopride was added to the buffer solution.
The films were analyzed using computer-assisted densitometry. Intensity levels within the region of interest (ROI) and the corpus callosum (used as a local background) were measured on a 10-point optical density calibration scale (Stouffer Graphic Arts Equipment, Mishawaka, IN). Then, these ROI intensity levels relative to the background intensity were averaged across the selected sections. Finally, the 125I plastic standards were also measured on a 10-point calibration scale and used to estimate the relative ROI intensity levels in femtomoles per milligram.
Statistical strategy
Prior to our analysis, we used Winsorizing (Dixon, 1960) to reduce the effect of any outliers; we found only one outlier of aggression (CA rat, z = 4.99) and set it to the closest nonextreme value; the other cases scored within z = ±3.0. In addition, one-way ANOVAs tested any group difference in background characteristics (i.e., age, aggressive behavior of the opponent rats, testosterone, and corticosterone).
To test our first hypothesis, a two-way ANOVA was performed to test the interaction effect between exposure length and exposure condition on the aggressive behavior of the observer rats. Moreover, three-way repeated measures ANOVAs were used to compare the D2R densities in the AcbC and AcbSh (at a Bonferroni-corrected significance level of p = .05/2), as well as the 5-HT1BR densities in the MeA, BMA, and BLA (at a Bonferroni-corrected significance level of p = .05/3), with hemisphere as a within-subjects variable and exposure length and exposure condition as between-subjects variables. Finally, if any interaction was significant in the ANOVAs, Bonferroni-corrected post hoc tests were used to test the pairwise differences. To test the second and third hypotheses, Pearson correlations and hierarchical regressions (with follow-up simple regressions, if necessary) were used to test the associations among the local receptor densities and aggressive behavior identified by the initial ANOVAs (at the first step) and any possible interactions (at higher-order steps).
Results
Effects of passive exposure to aggression
Age, aggression of the opponent rats, testosterone, and corticosterone did not differ across the groups (see Table 1). However, although there was no age difference between the groups statistically, age was entered as a covariate in subsequent analyses in case there might be a sensitive period in the development of aggression and/or the effect of social exposure during our experiments.
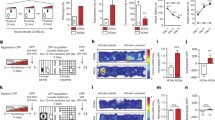
For testing aggressive behavior in the observer rats, we found significant main effects of exposure length [F(1, 67) = 11.51, p < .01] and exposure condition [F(1, 67) = 5.32, p < .05]. Furthermore, a significant interaction emerged between exposure length and exposure condition [F(1, 67) = 4.44, p < .05]. As Fig. 2 illustrates, the CA group showed more aggression than any of the other groups (p < .05), whereas there were no other pair-wise differences.
In addition, D2R density was examined in the nucleus accumbens of the observer rats. A main effect of exposure length was apparent [F(1, 67) = 21.57, corrected p < .01], and an interaction effect [F(1, 67) = 7.82, corrected p < .05] on D2R density in the AcbC. Specifically, the AA group showed higher D2R in the AcbC than any of the other groups (p < .05; see Fig. 3a). In addition, a main effect of exposure length [F(1, 67) = 32.45, corrected p < .01] and an interaction effect [F(1, 67) = 23.89, corrected p < .01] were found in D2R density in the AcbSh. Here, the CA group showed lower D2R in the AcbSh than any of the other groups (p < .01), and the AA group showed higher D2R than the CN group (p < .01; see Fig. 3b; for representative receptor densities, see Fig. 4.) Main effects of exposure condition and hemisphere, as well as any other interactions, were not found in these analyses.
Dopamine D2 receptor (D2R) ligand binding levels in the nucleus accumbens core (AcbC, shown in panel a) and shell (AcbSh, shown in panel b). AN = acute exposure to nonaggression; CN = chronic exposure to nonaggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. Error bars represent standard error of the mean. * p < .05, ** p < .01
Representative images of dopamine D2 receptor density in the nucleus accumbens. Darker gray indicates higher density. AN = acute exposure to nonaggression; CN = chronic exposure to nonaggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. The atlas drawings are obtained from Paxinos and Watson (2005)
5-HT1BR density was also examined in the amygdalae of the observer rats. A main effect of exposure condition was found in 5-HT1BR density in the BLA [F(1, 67) = 28.80, corrected p < .01]; the rats exposed to aggression (AA and CA rats) showed higher 5-HT1BR density than did the rats exposed to nonaggression (AN and CN rats; see Fig. 5c; for representative receptor densities, see Fig. 6.) In contrast, no main effects of exposure length and hemisphere, nor any interaction, were found.
5-HT1B receptor (5-HT1BR) ligand binding levels in the medial amygdala (MeA, shown in panel A), basomedial amygdala (BMA, shown in panel B), and basolateral amygdala (BLA, shown in panel C). AN = acute exposure to nonaggression; CN = chronic exposure to nonaggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. Error bars represent standard error of the mean. ** p < .01
Representative images of 5-HT1B receptor density in the amygdala. Darker gray indicates higher density. AN = acute exposure to nonaggression; CN = chronic exposure to nonaggression; AA = acute exposure to aggression; CA = chronic exposure to aggression. The atlas drawings are obtained from Paxinos and Watson (2005)
Associations among D2R and 5-HT1BR
The ANOVA results above identified three biomarkers for passive exposure to aggression: D2R densities in the AcbC and AcbSh, and 5-HT1BR density in the BLA. Thus, we further examined whether these three local receptor densities were correlated with each other. Because no effect of hemisphere emerged in the results above, each local receptor density value was averaged over the left and right hemispheres, to simplify our subsequent analyses and to control for Type II error rate.
Table 2 illustrates the results of partial correlations (with age as a covariate) with Bonferroni correction in pooled subjects, as well as within each group. In general, rats showed a significant positive correlation between D2R density in the AcbC and D2R density in the AcbSh. Nonetheless, no other partial correlations were found, although there were marginal correlations between (1) D2R density in the AcbC and 5-HT1BR density in the BLA among the acute exposure groups (p = .057), and (2) D2R density in the AcbSh and 5-HT1BR density in the BLA among the chronic exposure groups (p = .051).
D2R and 5-HT1BR densities in relation to aggressive behavior
We further used a hierarchical regression to predict aggressive behavior in the observer rats; the first step included all main effects of the identified receptor densities, the second step added all possible two-way interactions, and the third step added the three-way interaction (see Table 3). In pooled subjects, the first step [F(4, 67) = 4.84, p < .01] revealed that, with other variables constant, D2R densities in the AcbC and AcbSh, respectively, contributed to predicting aggressive behavior. Specifically, aggression increased as D2R density in the AcbC increased or as D2R density in the AcbSh decreased. In contrast, 5-HT1BR density in the BLA was not associated with aggression directly. The second step [F(7, 64) = 3.53, p < .01] showed that the two-way interaction between D2R density in the AcbSh and 5-HT1BR density in the BLA, but not the other two-way interactions, significantly contributed to predicting aggressive behavior. Finally, the third step [F(8, 63) = 3.04, p < .01] indicated that the three-way interaction was not a significant predictor of aggression. We also performed a simple regression analysis within each group using Bonferroni corrections. In these analyses, none of the effects identified above remained significant, although this might have been due to reduced statistical power. Therefore, aggressive behavior was associated with (1) D2R density in the AcbC and (2) a combination of D2R density in the AcbSh and 5-HT1BR density in the BLA, respectively, regardless of exposure to aggression.
To visualize the interaction between D2R density in the AcbSh and 5-HT1BR density in the BLA, Fig. 7 shows a scatterplot describing the association between D2R density in the AcbSh and aggression, moderated by three regression lines with different levels of 5-HT1BR density in the BLA. Each line represents the slope for aggression on D2R density in the AcbSh while 5-HT1BR density in the BLA was held at either a high value (centered around its mean plus one standard deviation), a middle value (centered around its mean), or a low value (centered around its mean minus one standard deviation). The solid straight line, representing the condition of high 5-HT1BR density in the BLA, indicates a stronger negative association between D2R density in the AcbSh and aggression (B = –.55, constant = 54.35) than do the dashed line, representing the condition of average 5-HT1BR density in the BLA (B = –.19, constant = 28.40), and the dotted line, representing the condition of low 5-HT1BR density in the BLA (B = .17, constant = 2.45). Therefore, aggression increased when D2R density in the AcbSh was low, especially when 5-HT1BR density in the BLA was high.
Scatterplot representing the relationship between aggressive behavior and dopamine D2 receptor (D2R) density in the shell of the nucleus accumbens, moderated by 5-HT1B receptor (5-HT1BR) density in the basolateral amygdala at a high value (solid line), middle value (dashed line), and low value (dotted line)
Discussion
The purpose of this study was to test our hypotheses that, in contrast to acute exposure, chronic exposure to aggression would lead observer rats to show (1) higher levels of impulsive aggression, (2) lower D2R density in the AcbSh, and (3) higher 5-HT1BR density in the BLA. We also hypothesized that D2R density would be associated with 5-HT1BR density, and it would predict impulsive aggressive behavior. Four major findings were obtained.
First, our results revealed that observer rats showed increased impulsive aggressive behavior only when they were passively exposed to aggressive situations chronically. In contrast, acute exposure to aggression did not increase impulsivity/aggressiveness in observer rats, as compared to chronic exposure to aggression. These findings exactly replicated those from a previous study (Suzuki & Lucas, 2010). Therefore, although a single-time observation of aggression does not necessarily lead to social learning of aggression in observers, repeated observation of aggression is a risk factor socializing observers to learn aggressive manners (Huesmann & Kirwil, 2007).
Second, all accumbal regions, regardless of hemisphere, generally showed lower D2R density in the chronic exposure conditions than in the acute exposure conditions, and this effect further depended on whether or not the observer rats were exposed to aggression. In particular, as compared with the nonaggression exposure control conditions, acute passive exposure to aggression increased D2R density in the AcbC, whereas chronic passive exposure to aggression downregulated D2R density in the AcbSh. These contrasting patterns may reflect that the AcbC and AcbSh actually have differential functions. For example, Bassareo, De Luca, and Di Chiara (2002) found that, although dopaminergic activity levels in both AcbC and AcbSh are activated by novel appetitive stimuli, only dopamine response in the AcbSh is then habituated and reduced following repeated appetitive stimuli. Thus, assuming that aggression has some rewarding properties (May & Kennedy, 2009), acute passive exposure to aggression may rapidly enhance dopaminergic activity by upregulating D2R density in the AcbC and AcbSh, as was seen in the AA group (see Fig. 3a). However, once dopaminergic activity became habituated by repeated exposure to aggression, this might abruptly reduce D2R density in the AcbSh (but not AcbC), as was seen in the CA group (see Fig. 3b). Future research needs to test this hypothesis.
Interestingly, a similar downregulated D2R density in the AcbSh has been found following chronic administration of cocaine (Moore, Vinsant, Nader, Porrino, & Friedman, 1998; Nader et al., 2002), morphine (Hemby, 2004), and anabolic–androgenic steroids (Kindlundh, Lindblom, Bergstrom, & Nyberg, 2003). On the other hand, other studies have addressed the issue that chronic use of psychostimulants induces extra release of dopamine in the nucleus accumbens (Hernandez & Hoebel, 1988; Weiss, Paulus, Lorang, & Koob, 1992). Taken together, high dopamine release may be correlated with low D2R density in the AcbSh, suggesting that downregulated D2R may result from a compensatory function to maintain dopamine activity. In the present study, chronic passive exposure to aggression may have produced effects on D2R similar to a long-term dose of psychostimulants, as indicated by the low D2R density in the CA group. Alternatively, the downregulation of D2R following chronic passive exposure to aggression may be subject to increased dopamine release in the AcbSh, which would be an intrinsically rewarding/salient signal for observer rats. In contrast, note that chronic stress is not associated with a compensatory downregulation of D2R density in the AcbSh immediately after stress (Lucas, Wang, McCall, & McEwen, 2007), or even after a recovery period (Lucas et al., 2004; Yohe et al., 2012). Accordingly, our findings were less likely to be confounded with any social stress effect (Tzanoulinou, Riccio, de Boer, & Sandi, 2014; Wommack & Delville, 2007). Indeed, no group difference was observed in the levels of serum corticosterone immediately following passive exposure (see Table 1).
The third major finding was that 5-HT1BR density in the BLA, but not in the other amygdaloid nuclei, was bilaterally upregulated in the observer rats exposed to aggression, and this finding was present regardless of exposure length. A previous study has reported that increased 5-HT1BR density in the BLA was identified following chronic passive exposure to aggression (Suzuki et al., 2010b), but our present results have extended these findings. That is, 5-HT1BR density in the BLA can be rapidly upregulated following even a single-time exposure to aggression. The subregional difference in 5-HT1BR density might explain some features of aggressive behavior in observer rats. For example, the MeA plays a role in emotion generation, such as fear-induced aggression (Siegel, Bhatt, Bhatt, & Zalcman, 2007), and has neural projections to the hypothalamus (Sah, Faber, Lopez De Armentia, & Power, 2003), which is essentially related to fearful and subordinate behavior in a social context (Motta et al., 2009). In contrast, passive exposure to aggression did not affect 5-HT1BR density in the MeA, and thus was presumably not related to self-defensive aggression or any fear-related aggression. Rather, passive exposure to aggression changed the structure of the BLA, which is involved in associative learning of emotions (e.g., emotional acquisition and conditioning) and shows neural projections to the striatum, nucleus accumbens, and prefrontal cortex (Sah et al., 2003). This suggests that exposure to aggression might initiate an emotional-learning process to make aggression accessible as the socio-behavioral repertoire. Further studies should clarify this hypothesis.
Finally, Table 3 shows that increased impulsive aggression was associated with (1) high D2R density in the AcbC, (2) low D2R density in the AcbSh, and (3) a combination of low D2R density in the AcbSh and high 5-HT1BR density in the BLA. However, our follow-up, simple regression analysis within each subgroup showed that these identified associations did not remain significant in the condition of passive exposure to aggression. A lack of findings in our subgroup analysis might have been due to the small group size (n = 18 each). Yet, at least, our results indicated that accumbal D2R and/or amygdaloid 5-HT1BR were generally linked with aggression, regardless of passive exposure to aggression. The positive association between impulsive aggression and D2R density in the AcbC was somewhat unexpected, because D2R density in the AcbC was actually lower in the CA group (which exclusively showed increased aggression) than in the AN and CN control groups. But, regardless of D2R levels in the AcbC, D2R density in the AcbSh showed a negative association with impulsive aggression, and this association was moderated by high 5-HT1BR density in the BLA (see Fig. 7). Because these behavioral and neurochemical outcomes resulted from chronic passive exposure to aggression, we propose that the interaction effect between D2R in the AcbSh and 5-HT1BR in the BLA on impulsive aggression provides a neurobiological perspective on why observers exposed to aggression chronically are at high risk for being aggressive. That is, chronic passive exposure to aggression downregulates D2R density in the AcbSh and upregulates 5-HT1BR density in the BLA among observers, and these neurochemical profiles are significantly associated with increased impulsive aggression.
Our findings on the interaction between the AcbSh and BLA may have some implications in the social learning of aggression. Generally, the BLA receives sensory inputs from the thalamus, hippocampus, and cortex (Davis & Whalen, 2001) and is involved in associative learning of emotional behavior (Sah et al., 2003), such as contextual fear conditioning (Fenton, Spicer, Halliday, Mason, & Stevenson, 2013; Herry et al., 2008; Maren, Poremba, & Gabriel, 1991) and social defeat conditioning (Morrison & Cooper, 2012). 5-HT1BR in the BLA is specifically associated with impulsive/aggressive trends, as is evident from a higher amount of binding of 5-HT1BR in pathologically aggressive animals than in normally behaving animals (Jacobs et al., 2007). In our paradigm, circumstances that provided an aggressive situation upregulated 5-HT1BR density in the BLA in passive observers. This may reflect associative learning of aggression, such that observer rats learned to associate an aggressive social interaction and its consequence (e.g., defeat) in a social encounter. Our behavioral results indeed demonstrated that repeatedly observing aggressive circumstances was necessary to reinforce observers’ aggressive responses in later social encounters. We expect that such reinforcing effects were probably related to D2R in the AcbSh because dopaminergic activity in the AcbSh, which is actually modulated by the BLA (Jackson & Moghaddam, 2001), plays an important role in motivational valence (i.e., aversive vs. rewarding; Bassareo et al., 2002; Jentsch & Taylor, 1999; Shirayama & Chaki, 2006). Interestingly, the intra-AcbSh infusion of a D2R antagonist, which acts to simulate low D2R availability, (1) switches an animal’s response from aversion to reward (Bernal et al., 2008; Laviolette, Lauzon, Bishop, Sun, & Tan, 2008), (2) disrupts the inhibitory control of hedonic behavior (Halpern et al., 2013), (3) increases appetitive social interaction (Thompson, Leonard, & Brudzynski, 2006), (4) facilitates self-administration of cocaine (Bachtell, Whisler, Karanian, & Self, 2005), and (5) increases impulsive behavior (Besson et al., 2010). Thus, low D2R in the AcbSh is related to high reward-seeking behavior. Furthermore, reduced D2R density could reflect a compensatory function for excessive dopamine release, which induces intrinsic rewards; although, to our knowledge, no studies have directly examined the relation between D2R and extracellular concentrations of dopamine, a number of separate studies on drug use have consistently shown that chronic use of psychostimulants results in low D2R density in the AcbSh (Hemby, 2004; Kindlundh et al., 2003; Moore et al., 1998; Nader et al., 2002) and excessive dopamine release in the nucleus accumbens (Hernandez & Hoebel, 1988; Weiss et al., 1992). On the basis of these findings, in our paradigm, repeatedly observing aggressive circumstances might accumulatively activate dopamine release in the nucleus accumbens. Consequently, D2R binding in the AcbSh was reduced as a compensatory function. Nevertheless, the drawback of the compensatory reduction of D2R density is that postsynaptic sensitivity to dopamine neurotransmission could be blunted if presynaptic dopamine release recovered to baseline. Accordingly, after being removed from chronic passive exposure to aggression, observer rats may experience blunted sensitivity to dopamine release (i.e., deficiency in dopamine-related rewards) and be motivated to fulfill their demands for dopamine. Their deficiency in dopamine may be treated by reward-seeking behavior, such as performing aggressive behavior (May & Kennedy, 2009). When all of the above environmental, psychological, and neurochemical factors are taken together, our results indicated that the combined effects of high 5-HT1BR density in the BLA (which may represent associative learning of aggression processed by exposure to aggression) and low D2R density in the AcbSh (which may represent the reinforcing and rewarding qualities of aggression being increased by repeated exposure to aggression) motivated observer rats to interact with a naïve rat aggressively.
Nevertheless, the following study limitations need to be noted: Although our findings indicated the linkage among aggressive behavior, D2R density, and 5-HT1BR density, there is still uncertainty with respect to the causal relationship among them. Moreover, it is still unclear whether age differences play a part in vulnerability to chronic exposure to aggression. On average, the postnatal day (P) in our sample of observer rats was specifically 44 days at the beginning of our exposure paradigm and 64 days at the time of assessing aggressive behavior. In a rat’s lifespan, P44 is around the late stage of periadolescence, and P64 is at the stage of young adulthood (Sengupta, 2013). A replication of our results may depend on the timing of being exposed to aggression (Mrug et al., 2014; Veenit, Cordero, Tzanoulinou, & Sandi, 2013) and/or the timing of the onset of aggression (Cleverley, Szatmari, Vaillancourt, Boyle, & Lipman, 2012; Hartup, 2005). More research will be needed to clarify the developmental vulnerability to chronic exposure to aggression.
In summary, for the present study we used a novel rat paradigm to examine the behavioral and neurochemical effects of passive exposure to aggression. Within this paradigm, it was demonstrated that chronic passive exposure to aggression increased impulsive aggressive behavior and reduced D2R density in the AcbSh among observer rats; in contrast, these effects were not found in acute exposure to aggression. In addition, as soon as observer rats were exposed to aggression, 5-HT1BR density in the BLA also increased. Furthermore, we also found that a combination of low D2R density in the AcbSh and high 5-HT1BR density in the BLA was associated with a high risk for impulsivity/aggressiveness. Overall, we concluded that repeated observations of aggression promote a number of neurobiological effects by downregulating D2R density in the AcbSh and upregulating 5-HT1B in the BLA, whereby observers are inclined to show increased impulsive aggression.
References
Anderson, C. A., & Huesmann, L. R. (2003). Human aggression: A social-cognitive view. In M. A. Hogg & J. C. Cooper (Eds.), The Sage handbook of social psychology (pp. 296–323). Thousand Oaks: Sage.
Arrant, A. E., Jemal, H., & Kuhn, C. M. (2013). Adolescent male rats are less sensitive than adults to the anxiogenic and serotonin-releasing effects of fenfluramine. Neuropharmacology, 65, 213–222. doi:10.1016/j.neuropharm.2012.10.010
Bachtell, R. K., Whisler, K., Karanian, D., & Self, D. W. (2005). Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology, 183, 41–53. doi:10.1007/s00213-005-0133-1
Bandura, A. (1973). Aggression: A social learning analysis. Englewood Cliffs: Prentice-Hall.
Bandura, A. (1977). Social learning theory. Englewood Cliffs: Prentice-Hall.
Bandura, A., Ross, D., & Ross, S. A. (1961). Transmission of aggression through imitation of aggressive models. Journal of Abnormal and Social Psychology, 63, 575–582.
Bandura, A., Ross, D., & Ross, S. A. (1963). Imitation of film-mediated agressive models. Journal of Abnormal and Social Psychology, 66, 3–11.
Bassareo, V., De Luca, M. A., & Di Chiara, G. (2002). Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. Journal of Neuroscience, 22, 4709–4719.
Bauer, N. S., Herrenkohl, T. I., Lozano, P., Rivara, F. P., Hill, K. G., & Hawkins, J. D. (2006). Childhood bullying involvement and exposure to intimate partner violence. Pediatrics, 118, e235–e242. doi:10.1542/peds. 2005-2509
Beiderbeck, D. I., Reber, S. O., Havasi, A., Bredewold, R., Veenema, A. H., & Neumann, I. D. (2012). High and abnormal forms of aggression in rats with extremes in trait anxiety—Involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology, 37, 1969–1980. doi:10.1016/j.psyneuen.2012.04.011
Berenson, A. B., Wiemann, C. M., & McCombs, S. (2001). Exposure to violence and associated health-risk behaviors among adolescent girls. Archives of Pediatrics and Adolescent Medicine, 155, 1238–1242.
Bernal, S. Y., Dostova, I., Kest, A., Abayev, Y., Kandova, E., Touzani, K., & Bodnar, R. J. (2008). Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor–flavor preferences in rats. Behavioural Brain Research, 190, 59–66. doi:10.1016/j.bbr.2008.02.003
Berridge, K. C. (2007). The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology, 191, 391–431. doi:10.1007/s00213-006-0578-x
Besson, M., Belin, D., McNamara, R., Theobald, D. E., Castel, A., Beckett, V. L., & Dalley, J. W. (2010). Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology, 35, 560–569. doi:10.1038/npp.2009.162
Blanchard, D. C., & Blanchard, R. J. (1990). Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neuroscience & Biobehavioral Reviews, 14, 455–462.
Blanchard, D. C., Sakai, R. R., McEwen, B., Weiss, S. M., & Blanchard, R. J. (1993). Subordination stress: behavioral, brain, and neuroendocrine correlates. Behavioural Brain Research, 58, 113–121.
Blanchard, D. C., Spencer, R. L., Weiss, S. M., Blanchard, R. J., McEwen, B., & Sakai, R. R. (1995). Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology, 20, 117–134.
Blanchard, R. J., Yudko, E., Dulloog, L., & Blanchard, D. C. (2001). Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiology & Behavior, 72, 635–642. doi:10.1016/S0031-9384(00)00449-2
Bustamante, D., Quintanilla, M. E., Tampier, L., Gonzalez-Lira, V., Israel, Y., & Herrera-Marschitz, M. (2008). Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. European Journal of Pharmacology, 591, 153–158. doi:10.1016/j.ejphar.2008.06.069
Cannon, W. B. (1939). The wisdom of the body. New York: Norton.
Caramaschi, D., de Boer, S. F., de Vries, H., & Koolhaas, J. M. (2008). Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behavioural Brain Research, 189, 263–272. doi:10.1016/j.bbr.2008.01.003
Cleverley, K., Szatmari, P., Vaillancourt, T., Boyle, M., & Lipman, E. (2012). Developmental trajectories of physical and indirect aggression from late childhood to adolescence: Sex differences and outcomes in emerging adulthood. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1037–1051. doi:10.1016/j.jaac.2012.07.010
Clotfelter, E. D., & Paolino, A. D. (2003). Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Animal Behaviour, 66, 343–347. doi:10.1006/Anbe.2003.2227
Couppis, M. H., & Kennedy, C. H. (2008). The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology, 197, 449–456. doi:10.1007/S00213-007-1054-Y
Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34.
Desai, R. I., Paronis, C. A., Martin, J., Desai, R., & Bergman, J. (2010). Monoaminergic psychomotor stimulants: discriminative stimulus effects and dopamine efflux. Journal of Pharmacology and Experimental Therapeutics, 333, 834–843. doi:10.1124/jpet.110.165746
Dixon, W. J. (1960). Simplified estimation from censored normal samples. Annals of Mathematical Statistics, 31, 269–556. doi:10.1214/aoms/1177705900
Emery, C. R. (2011). Controlling for selection effects in the relationship between child behavior problems and exposure to intimate partner violence. Journal of Interpersonal Violence, 26, 1541–1558. doi:10.1177/0886260510370597
Fantuzzo, J. W., DePaola, L. M., Lambert, L., Martino, T., Anderson, G., & Sutton, S. (1991). Effects of interparental violence on the psychological adjustment and competencies of young children. Journal of Consulting and Clinical Psychology, 59, 258–265.
Feldker, D. E., Morsink, M. C., Veenema, A. H., Datson, N. A., Proutski, V., Lathouwers, D., & Vreugdenhil, E. (2006). The effect of chronic exposure to highly aggressive mice on hippocampal gene expression of non-aggressive subordinates. Brain Research, 1089, 10–20. doi:10.1016/j.brainres.2006.02.110
Fenton, G. E., Spicer, C. H., Halliday, D. M., Mason, R., & Stevenson, C. W. (2013). Basolateral amygdala activity during the retrieval of associative learning under anesthesia. Neuroscience, 233, 146–156. doi:10.1016/j.neuroscience.2012.12.039
Ferrari, P. F., van Erp, A. M., Tornatzky, W., & Miczek, K. A. (2003). Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. European Journal of Neuroscience, 17, 371–378.
Ferris, C. F., Melloni, R. H., Jr., Koppel, G., Perry, K. W., Fuller, R. W., & Delville, Y. (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience, 17, 4331–4340.
Ferris, C. F., Stolberg, T., & Delville, Y. (1999). Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus). Behavioral Neuroscience, 113, 804–815.
Ferris, C. F., Stolberg, T., Kulkarni, P., Murugavel, M., Blanchard, R., Blanchard, D. C., & Simon, N. G. (2008). Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neuroscience, 9, 111. doi:10.1186/1471-2202-9-111
Guerra, N. G., Huesmann, L. R., & Spindler, A. (2003). Community violence exposure, social cognition, and aggression among urban elementary school children. Child Development, 74, 1561–1576.
Guerra, N. G., Huesmann, L. R., Tolan, P. H., Van Acker, R., & Eron, L. D. (1995). Stressful events and individual beliefs as correlates of economic disadvantage and aggression among urban children. Journal of Consulting and Clinical Psychology, 63, 518–528.
Halpern, C. H., Tekriwal, A., Santollo, J., Keating, J. G., Wolf, J. A., Daniels, D., & Bale, T. L. (2013). Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. Journal of Neuroscience, 33, 7122–7129. doi:10.1523/JNEUROSCI. 3237-12.2013
Hartup, W. W. (Ed.). (2005). The development of aggression. New York: Guilford Press.
Hemby, S. E. (2004). Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience, 126, 689–703. doi:10.1016/j.neuroscience.2004.01.056
Hernandez, L., & Hoebel, B. G. (1988). Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences, 42, 1705–1712.
Herry, C., Ciocchi, S., Senn, V., Demmou, L., Muller, C., & Luthi, A. (2008). Switching on and off fear by distinct neuronal circuits. Nature, 454, 600–606. doi:10.1038/nature07166
Holmes, M. R. (2013). The sleeper effect of intimate partner violence exposure: long-term consequences on young children’s aggressive behavior. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54, 986–995. doi:10.1111/jcpp. 12071
Huesmann, L. R., & Kirwil, L. (2007). Why observing violence increases the risk of violent behavior by the observer. In D. J. Flannery, A. T. Vazsonyi, & I. D. Waldman (Eds.), The Cambridge handbook of violent behavior and aggression (pp. 545–570). New York: Cambridge University Press.
Jackson, M. E., & Moghaddam, B. (2001). Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. Journal of Neuroscience, 21, 676–681.
Jacobs, C., Van Den Broeck, W., & Simoens, P. (2007). Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Research, 1136, 102–109. doi:10.1016/j.brainres.2006.11.096
Jentsch, J. D., & Taylor, J. R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology, 146, 373–390.
Kilpatrick, D. G., Acierno, R., Saunders, B., Resnick, H. S., Best, C. L., & Schnurr, P. P. (2000). Risk factors for adolescent substance abuse and dependence: Data from a national sample. Journal of Consulting and Clinical Psychology, 68, 19–30.
Kindlundh, A. M., Lindblom, J., Bergstrom, L., & Nyberg, F. (2003). The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience, 119, 113–120.
Kleijn, J., Wiskerke, J., Cremers, T. I., Schoffelmeer, A. N., Westerink, B. H., & Pattij, T. (2012). Effects of amphetamine on dopamine release in the rat nucleus accumbens shell region depend on cannabinoid CB1 receptor activation. Neurochemistry International, 60, 791–798. doi:10.1016/j.neuint.2012.03.002
Kline, P. (1999). The handbook of psychological testing (2nd ed.). London: Routledge.
Koolhaas, J. M., de Boer, S. F., Buwalda, B., & van Reenen, K. (2007). Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain, Behavior and Evolution, 70, 218–226. doi:10.1159/000105485
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., & Blokhuis, H. J. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews, 23, 925–935.
Koolhaas, J. M., Meerlo, P., De Boer, S. F., Strubbe, J. H., & Bohus, B. (1997). The temporal dynamics of the stress response. Neuroscience & Biobehavioral Reviews, 21, 775–782.
Laviolette, S. R., Lauzon, N. M., Bishop, S. F., Sun, N., & Tan, H. (2008). Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. Journal of Neuroscience, 28, 8025–8033. doi:10.1523/JNEUROSCI. 1371-08.2008
Lucas, L. R., Celen, Z., Tamashiro, K. L., Blanchard, R. J., Blanchard, D. C., Markham, C., & McEwen, B. S. (2004). Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience, 124, 449–457. doi:10.1016/j.neuroscience.2003.12.009
Lucas, L. R., Wang, C. J., McCall, T. J., & McEwen, B. S. (2007). Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Research, 1155, 108–115. doi:10.1016/j.brainres.2007.04.063
Maren, S., Poremba, A., & Gabriel, M. (1991). Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Research, 549, 311–316.
Margolin, G., & Gordis, E. B. (2000). The effects of family and community violence on children. Annual Review of Psychology, 51, 445–479. doi:10.1146/annurev.psych.51.1.445
May, M. E., & Kennedy, C. H. (2009). Aggression as positive reinforcement in mice under various ratio- and time-based reinforcement schedules. Journal of the Experimental Analysis of Behavior, 91, 185–196. doi:10.1901/Jeab. 2009.91-185
Miczek, K. A. (1974). Intraspecies aggression in rats: Effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia, 39, 275–301.
Moore, R. J., Vinsant, S. L., Nader, M. A., Porrino, L. J., & Friedman, D. P. (1998). Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse, 30, 88–96. doi:10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L
Morrison, K. E., & Cooper, M. A. (2012). A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacology, Biochemistry, and Behavior, 100, 592–600. doi:10.1016/j.pbb.2011.09.005
Motta, S. C., Goto, M., Gouveia, F. V., Baldo, M. V., Canteras, N. S., & Swanson, L. W. (2009). Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proceedings of the National Academy of Sciences, 106, 4870–4875. doi:10.1073/pnas.0900939106
Mrug, S., Elliott, M. N., Davies, S., Tortolero, S. R., Cuccaro, P., & Schuster, M. A. (2014). Early puberty, negative peer influence, and problem behaviors in adolescent girls. Pediatrics, 133, 7–14. doi:10.1542/peds. 2013-0628
Nader, M. A., Daunais, J. B., Moore, T., Nader, S. H., Moore, R. J., Smith, H. R., & Porrino, L. J. (2002). Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology, 27, 35–46. doi:10.1016/S0893-133X(01)00427-4
Nelson, R. J., & Trainor, B. C. (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience, 8, 536–546. doi:10.1038/nrn2174
Paxinos, G., & Watson, C. (2005). The rat brain in stereotaxic coordinates (5th ed.). San Diego: Elsevier Academic Press.
Pellis, S. M., & Pellis, V. C. (1987). Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat rattus norvegicus. Aggressive Behavior, 13, 227–242.
Pellis, S. M., Pellis, V. C., & Foroud, A. (2005). Play fighting: Aggression, affiliation, and the development of nuanced social skills. In R. E. Tremblay, W. W. Hartup, & J. Archer (Eds.), Developmental origins of aggression (pp. 47–62). New York: Guilford.
Pihl, R. O., & Benkelfat, C. (Eds.). (2005). Neuromodulators in the development and expression of inhibition and aggression. New York: Guilford Press.
Sah, P., Faber, E. S. L., Lopez De Armentia, M., & Power, J. (2003). The amygdala complex: Anatomy and physiology. Physiological Reviews, 83, 803–834. doi:10.1152/physrev.00002.2003
Sengupta, P. (2013). The laboratory rat: Relating its age with human’s. International Journal of Preventive Medicine, 4, 624–630.
Shirayama, Y., & Chaki, S. (2006). Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Current Neuropharmacology, 4, 277–291.
Siegel, A., Bhatt, S., Bhatt, R., & Zalcman, S. S. (2007). The neurobiological bases for development of pharmacological treatments of aggressive disorders. Current Neuropharmacology, 5, 135–147.
Su, W., Mrug, S., & Windle, M. (2010). Social cognitive and emotional mediators link violence exposure and parental nurturance to adolescent aggression. Journal of Clinical Child and Adolescent Psychology, 39, 814–824. doi:10.1080/15374416.2010.517163
Sussman, S., Dent, C. W., & McCullar, W. J. (2000). Group self-identification as a prospective predictor of drug use and violence in high-risk youth. Psychology of Addictive Behaviors, 14, 192–196.
Sussman, S., Dent, C. W., & Stacy, A. W. (1999). The association of current stimulant use with demographic, substance use, violence-related, social and intrapersonal variables among high risk youth. Addictive Behaviors, 24, 741–748.
Suzuki, H., Han, S. D., & Lucas, L. R. (2010a). Chronic passive exposure to aggression decreases D(2) and 5-HT(1B) receptor densities. Physiology & Behavior, 99, 562–570. doi:10.1016/j.physbeh.2010.01.018
Suzuki, H., Han, S. D., & Lucas, L. R. (2010b). Increased 5-HT1B receptor density in the basolateral amygdala of passive observer rats exposed to aggression. Brain Research Bulletin, 83, 38–43. doi:10.1016/j.brainresbull.2010.06.007
Suzuki, H., & Lucas, L. R. (2010). Chronic passive exposure to aggression escalates aggressiveness of rat observers. Aggressive Behavior, 36, 54–66. doi:10.1002/ab.20333
Tamashiro, K. L., Nguyen, M. M., & Sakai, R. R. (2005). Social stress: From rodents to primates. Frontiers in Neuroendocrinology, 26, 27–40. doi:10.1016/j.yfrne.2005.03.001
Thompson, B., Leonard, K. C., & Brudzynski, S. M. (2006). Amphetamine-induced 50 kHz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behavioural Brain Research, 168, 64–73. doi:10.1016/j.bbr.2005.10.012
Tran, L., Lasher, B. K., Young, K. A., & Keele, N. B. (2013). Depletion of serotonin in the basolateral amygdala elevates glutamate receptors and facilitates fear-potentiated startle. Translational Psychiatry, 3, e298. doi:10.1038/tp.2013.66
Tzanoulinou, S., Riccio, O., de Boer, M. W., & Sandi, C. (2014). Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational Psychiatry, 4, e410. doi:10.1038/tp.2014.54
van Erp, A. M., & Miczek, K. A. (2000). Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. Journal of Neuroscience, 20, 9320–9325.
van Erp, A. M., & Miczek, K. A. (2007). Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology, 191, 679–688. doi:10.1007/s00213-006-0637-3
Veenit, V., Cordero, M. I., Tzanoulinou, S., & Sandi, C. (2013). Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Frontiers in Behavioral Neuroscience, 7, 26. doi:10.3389/fnbeh.2013.00026
Vermeiren, R., Schwab-Stone, M., Deboutte, D., Leckman, P. E., & Ruchkin, V. (2003). Violence exposure and substance use in adolescents: Findings from three countries. Pediatrics, 111, 535–540.
Vitiello, B., & Stoff, D. M. (1997). Subtypes of aggression and their relevance to child psychiatry. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 307–315. doi:10.1097/00004583-199703000-00008
Weiss, F., Paulus, M. P., Lorang, M. T., & Koob, G. F. (1992). Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: Effects of acute and repeated administration. Journal of Neuroscience, 12, 4372–4380.
Welch, A. S., & Welch, B. L. (1971). Isolation, reactivity and aggression: Evidence for an involvement of brain catecholamines and serotonin. In B. E. Eleftheriou & J. P. Scott (Eds.), The physiology of aggression and defeat: Proceedings of a symposium held during the meeting of the American Association for the Advancement of Science in Dallas, Texas, December 1968 (pp. 91–142). New York: Plenum Press.
Widom, C. S. (1989). Does violence beget violence? A critical examination of the literature. Psychological Bulletin, 106, 3–28. doi:10.1037/0033-2909.106.1.3
Wommack, J. C., & Delville, Y. (2007). Stress, aggression, and puberty: Neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain, Behavior and Evolution, 70, 267–273. doi:10.1159/000105490
Wood, G. E., Norris, E. H., Waters, E., Stoldt, J. T., & McEwen, B. S. (2008). Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behavioral Neuroscience, 122, 282–292. doi:10.1037/0735-7044.122.2.282
Wood, G. E., Young, L. T., Reagan, L. P., & McEwen, B. S. (2003). Acute and chronic restraint stress alter the incidence of social conflict in male rats. Hormones and Behavior, 43, 205–213.
Yohe, L. R., Suzuki, H., & Lucas, L. R. (2012). Aggression is suppressed by acute stress but induced by chronic stress: immobilization effects on aggression, hormones, and cortical 5-HT(1B)/striatal dopamine D(2) receptor density. Cognitive, Affective, & Behavioral Neuroscience, 12, 446–459. doi:10.3758/s13415-012-0095-9
Author note
The authors report no conflicts of interests. This work was supported by a Loyola University Chicago Research Support grant (to L.R.L). H.S.’s time was supported by NIH Grant Nos. MH090786 and MH098099.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, H., Lucas, L.R. Neurochemical correlates of accumbal dopamine D2 and amygdaloid 5-HT1B receptor densities on observational learning of aggression. Cogn Affect Behav Neurosci 15, 460–474 (2015). https://doi.org/10.3758/s13415-015-0337-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-015-0337-8