Abstract
The space surrounding the body in which individuals interact with the environment is known as the peripersonal space (PPS). Previous studies have reported that PPS has multisensory nature. However, the relationship between the multisensory nature of PPS and an individuals’ defensive actions has not been fully clarified to date. We investigated this relationship by examining the multisensory representation of PPS under situations in which visual feedback of body movements was delayed by using a virtual reality system. The results indicated that body-movement delays extended the multisensory PPS, suggesting that body-movement delays increased the potential threat of distant objects because it was necessary to prepare defensive actions sooner. The previous findings can be interpreted that PPS is modulated by the spatio-temporal relationship between people and external stimuli. This view may provide evidence of interactions between defensive and nondefensive functions of the multisensory PPS.
Similar content being viewed by others
Introduction
The peripersonal space (PPS) is the space surrounding the body, in which people physically interact with the environment through body movement (Rizzolatti et al., 1997). PPS is encoded differently from other spatial representations by multisensory neurons in the frontoparietal area, especially in the ventral premotor cortex (vPMC) and the intraparietal sulcus (IPS). Previous studies have suggested that PPS supports defensive action (for review, see de Vignemont & Iannetti, 2015; Graziano & Cooke, 2006; Hunley & Lourenco, 2018) represented by “fight or flight” instincts. When humans and other animals encounter potentially threatening objects, they choose to fight or maintain their distance from the threatening object by keeping them away from their safe area. People also take defensive action such as dodging, evading, or catching to prevent even an intrinsically nonthreatening object coming close to their body from hitting them, which is essential for their survival.
Behavioral studies have shown that PPS is related to defensive functions. The hand blink reflex (HBR) is a defensive reflex eye blink response observed when a hand presented in front of the face is electrically stimulated. Sambo et al. (2012) showed that HBR is stronger when the hand is presented near the face than when the hand is presented far from the face. HBR has been used as a measurement of face-centered defensive PPS because of this spatially dependent response. However, it remains unknown whether neural mechanisms of HBR are included or are related to the multisensory frontoparietal network of PPS. Then, HBR might rely on a mechanism other than the multisensory PPS (for a review, see Hunley & Lourenco, 2018; Serino, 2019).
There is some evidence that trait anxiety might affect the size of PPS. Lourenco et al. (2011) demonstrated that individuals with high claustrophobic traits have a larger PPS, and Sambo and Iannetti (2013) indicated that individual variability in trait anxiety is related to individual differences in PPS extension, although the study showed no relationship between PPS and claustrophobia. Similarly, Taffou and Viaud-Delmon (2014) demonstrated that participants with high cynophobia show extended multisensory representations of PPS when hearing a barking dog.
We focused on whether adaptation to temporally delayed movements in a virtual environment affects the multisensory system of PPS. Objects further away might become a threat when body movements are delayed than when they are not because people must act sooner to dodge, knockdown, or catch distant objects. If PPS supports defensive movements, PPS might become extended when a person’s movements are delayed. Most studies investigating defensive PPS have adapted HBR tasks, which, as described above, might rely on different mechanisms from the multisensory PPS. Therefore, we used a different multisensory task developed by Serino and his colleagues (e.g., Serino et al., 2017) that only a few studies have used for investigating the effect of threatening stimuli on PPS (Poliakoff et al., 2007; Taffou & Viaud-Delmon, 2014). Taffou and Viaud-Delmon (2014) demonstrated PPS extensions in a multisensory task with participants having high cynophobia using auditory stimuli of a barking dog. Previous studies have reported that approaching images of threatening stimuli are judged to arrive sooner than normal stimuli (Vagnoni et al., 2012; Vagnoni et al., 2015). This finding suggests that the location of threatening stimuli might be perceived closer than normal stimuli because threatening stimuli are perceived sooner (i.e., the modulation of the perception of threatening stimuli itself, but not PPS boundaries). Threatening stimuli are also more salient than normal stimuli. Therefore, saliency differences might affect reaction times to visuo-tactile or audio-tactile stimuli. As a result, the use of threatening stimuli in multisensory tasks might involve several confounding factors. This study used a neutral object (a white ball) as a visual stimulus. As described above, humans must be prepared to take specific actions to prevent an object coming close to our body from hitting us. We hypothesized that if PPS has a defensive function, body-movement delays will extend PPS even if the approaching visual stimulus is unthreatening.
Methods
Participants
We recruited healthy, right-handed, paid volunteers with normal or corrected-to-normal stereo vision as participants (N = 20, mean age, 20.7 years; age range: 19–25 years). The sample size was derived from the power analysis of previous studies (Noel et al., 2015; Taffou & Viaud-Delmon, 2014). They gave their written informed consent for participation before the experiments. The experiments and all the procedures were approved by the ethics committee of the Department of Psychology at the University of Tokyo. The experiments were conducted according to the principles and guidelines of the Declaration of Helsinki.
Apparatus and stimuli
Participants were shown a virtual environment from a first-person perspective through a head-mounted display (HTC VIVE, displaying a stereoscopic image at a resolution of 2,160 × 1,200). A right-hand virtual avatar moved in the virtual environment, either synchronously or after an approximate 440-ms delay from the participant's right-hand movements. The virtual world was developed using Unity3D and run on a Windows PC (Level Infinity by iiyama: Intel core i7-7700HQ at 2.8 GHz, 16 GB RAM, and NVIDIA GeForce GTX 1060), and the participants’ hand movements were tracked using an HTC VIVE controller.
The visual stimulus was a white ball having a 10-cm diameter shown in the virtual environment. It originated 300 cm from the participants and approached the participants at a velocity of 75 cm/s. The tactile stimulus consisted of vibrations of 80 Hz for 200 ms delivered from the controller held in the participant’s right hand. The controller itself vibrated. Therefore, the controller provided the tactile stimulus to a participant's entire hand rather than to a specific part of the hand.
Procedure
The experiment was split and conducted over two days. The participants performed a unisensory task, an adaptation task, and a multisensory task each day. The virtual right-hand avatar’s movements were perfectly synchronized with the participants’ right-hand movements during the adaptation and the multisensory tasks (no-delay condition) on one of the two days, whereas there was a delay of approximately 440 ms (the delay condition) on the other day. Half of the participants were assigned to the No-Delay condition on the first day and the Delay condition on the second day, whereas the other half were vice versa. In contrast, the unisensory task was identical on both days.
The participants sat at a table in a laboratory. They were instructed to adjust the head-mounted display and hold a controller in the right hand. Before starting the experiment, they placed their chin on a chin rest attached to the table to prevent head movements. Then, the virtual right-hand avatar was presented in the virtual environment. The participants first performed the unisensory task in which the ball was presented 300 cm in front of them, which disappeared when the experimenter pressed a button. After the ball disappeared, only the tactile stimulus was introduced to their right hand following a randomized interval of 1.53 s, 1.93 s, 2.33 s, 2.73 s, or 3.13 s. The participants reacted to the tactile stimulus by pressing a button with their left index fingers. The reaction time to the unisensory (tactile) stimulus was considered the baseline reaction time of each participant. The unisensory task consisted of 24 trials, including four catch trials in which no tactile stimuli were presented.
After the unisensory task, the participants performed the adaptation task, in which a cube repeatedly moved from side to side in the virtual environment facing the participants. The participants were instructed to reach for the cube as accurately as possible with the right-hand avatar. Before the adaptation task started, the participants were instructed to extend their arm forward, then the cube position was adjusted to the hand position. This procedure was done to reduce the variabilities of the reaching direction between participants because this study focused on the extension of PPS in the forward direction only. The participants could reach the target by moving their arms in oblique directions if the distance to the target were short enough because the target cube was moving from side to side facing the participants. The cube disappeared when the hand avatar touched the cube, and the subsequent trial was initiated. The adaptation task consisted of 144 trials that participants completed in an average of 15 minutes. (Neither moving kinematics nor reaching scores for the task were recorded.)
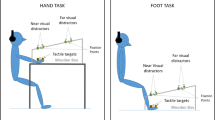
Finally, the participants performed the multisensory task (see Fig. 1), in which each trial was divided into an adaptation task and a multisensory stimuli reaction task. After a single adaptation task trial, the visual stimulus (the white ball) appeared 300 cm in front of the participants and approached them at a velocity of 75 m/s when the experimenter pressed a button. The tactile stimulus was also presented for either 1.53 s, 1.93 s, 2.33 s, 2.73 s, or 3.13 s after the onset of the visual stimulus. The tactile stimulus presentation onset corresponded to the spatial dimensions of the visual stimulus at 185 cm (D1), 155 cm (D2), 125 cm (D3), 95 cm (D4), or 65 cm (D5), respectively. Participants were instructed to react to the tactile stimulus by pressing a button as fast as possible with their left index finger while ignoring the visual stimuli. The screen went dark after a participant pressed the button, and the subsequent trial was initiated. The participants performed 120 trials: visual stimulus position [D1 to D5] × 20 repetitions and 20 catch trials with no tactile stimuli.
The multisensory task setup in the virtual environment. Participants were first instructed to reach for a target (a red cube) with the hand avatar. The white ball appeared after the hand avatar touched the target, which then approached the participants. The tactile stimulus was delivered to the participants’ right hand when the ball was either at D1, D2, D3, D4, or D5. The time shown next to each visual stimulus position indicates the time when the tactile stimulus was delivered after the white ball started approaching. (Color figure online)
Results
The multisensory facilitation effect was defined as the difference between the reaction time to the unisensory stimulus (no visual stimulus) and the reaction time to multisensory stimuli. A negative value indicates the facilitation of the reaction to tactile stimuli caused by presenting the visual stimuli. The multisensory facilitation effect was calculated for each participant under each condition. We defined outliers as reaction times exceeding 1,000 ms or three times the standard deviation of each participant’s mean reaction time under each condition, excluded from the analysis (2.9% of trials). All analyses were conducted using the statistical software Package R. Figure 2 displays the results of Experiment 1.
A two-way repeated-measures analysis of variance (ANOVA) indicated a significant main effect of the visual stimulus position, F(4, 76) = 54.31, p < .001, ηp = .741, and a significant interaction between the delay and visual stimulus distance, F(4, 76) = 2.54, p = .0468, ηp = .118. However, the main effect of the delay was not significant, F(1, 19) = 2.41, p = .137, ηp = .113.
We tested the simple main effect of delay at each visual stimulus position to identify the source of the interaction between the delay and the positions of the visual stimulus, which indicated that the multisensory facilitation effect was significantly higher at D1 and D2 in the delay condition than in the no-delay condition—D1: F(1, 19) = 5.51, p = .0299, ηp = .225; D2: F (1, 19) = 4.49, p = .0474, ηp = .191. However, there was no significant multisensory facilitation effect at D3, D4, or D5 in the delay condition compared with the no-delay condition—D3: F(1, 19) = 0.51, p = .480, ηp = .027; D4: F(1, 19) = 1.95, p = .178, ηp = .093; D5: F(1, 19) = 0.964, p = .339, ηp = .048. A post hoc Holm’s corrected multiple comparison tests (corrected for five comparisons in each condition) indicated that participants in the no-delay condition had significantly higher multisensory facilitation effect compared with zero (i.e., faster responses to multisensory stimuli than to unisensory stimuli) at D2, D3, D4, and D5, but not at D1—D1: t(19) = 0.519, d = 0.12, p = .610; D2: t(19) = 3.81, d = 0.85, p = .0012; D3: t(19) = 5.75, d = 1.29, p < .001; D4: t(19) = 6.21, p < .001, d = 1.39; D5: t(19) = 5.14, p < .001, d = 1.15. Conversely, participants showed higher multisensory facilitation effect compared with zero at all visual stimulus positions in the delay condition—D1: t(19) = 2.98, p = .0078, d = 0.67; D2: t(19) = 6.17, p < .001, d = 1.38; D3: t(19) = 6.64, p < .001, d = 1.49; D4: t(19) = 7.24, p < .001, d = 1.62; D5: t(19) = 5.45, p < .001 d = 1.22.
Discussion
This study demonstrated higher multisensory facilitation in far spaces when participants adapted to delayed movements than to no delay was presented, suggesting that the multisensory PPS representation extended after the adaptation to body-movement delays. A model in which PPS is divided into defensive and working PPS at functional, sensory, and motor levels was developed by de Vignemont and Iannetti (2015). They suggested that the working PPS, sometimes called “the reaching space,” is more closely related to goal-directed actions, whereas the defensive PPS, sometimes referred to as “the safety margin,” is more closely associated with its protective actions.
We demonstrated an extension of the PPS caused by a body movement delay. To what extent did the PPS extension observed in this study reflect defensive functions? It is possible that PPS extension in this study did not reflect defensive functions at all. No threatening objects were used as visual stimuli in this study because threatening stimuli might introduce confounding variables, such as saliency and time perception distortions. As a result, we ignored the role of emotions in this experiment, which might have limited the study’s findings because defensive actions often involve specific emotional states such as fear, anxiety, anger, and excitement. Several studies have shown that emotions affect spatial perception and attention (for a review, see Zadra & Clore, 2011). However, body movement delay has more significant consequences in defensive than in nondefensive situations because body movement delay affects the onset of action. People are forced to detect approaching potential threats sooner (i.e., at a more distant location) and begin protective actions earlier because of body-movement delays. Moreover, body-movement delays affect the onset of a motion, but not the spatial limits of participants’ activities with the body (such as reaching). Thus, action delays are crucial when reacting to approaching stimuli than to static stimuli. Our findings might be related to more global functions of PPS in guiding actions when reacting to approaching objects, which can be defensive or nondefensive, rather than being specifically related to the defensive PPS. However, defensive responses to approaching, nonthreatening stimuli have been observed in various animal species, including humans (Schiff, 1965), suggesting that merely approaching the body might lead to defensive actions. Therefore, findings of this study have substantial implications for the defensive functions of PPS.
Body schema, composed of visual and proprioceptive inputs, provides spatial information about the body’s or body part’s position in external space. Many studies have examined spatial overlaps between PPS and body schema (for a review, see Cardinali et al., 2009); on the other hand, several studies have implied a dissociation between PPS and body schema. A remotely controlled mouse cursor or a virtual-hand avatar can modulate PPS representation (Bassolino et al., 2010; Mine & Yokosawa, 2021a, 2021b). However, it is unclear whether body schema changes when using tools or avatars disconnected from the body. The current findings could be evidence of the dissociation between PPS and body schema. Serino et al. (2015) demonstrated that synchronous tactile stimuli delivered to a participant’s hand and auditory stimuli presented in far space leads to PPS extensions without using tools. Noel et al. (2015) showed that walking on a treadmill extends PPS into the forward space. Likewise, Amemiya et al. (2019) indicated that the walking-like sensations induced by vibrations to the sole extend PPS into the forward space. These studies have assumed that body schema does not change because the participants’ body or body parts did not move forward, although PPS was extended. The present findings also provide evidence of a dissociation between PPS and body schema because the action delay manipulation did not extend the limitation of reaching space, and it was assumed that body schema did not extend to the forward space, even though PPS was extended. However, neither the previous studies described above, nor the present study measured body schema. We suggest that future works should directly compare PPS and body schema under identical conditions to this study.
The relationship between body ownership and PPS has been investigated in several studies (see Makin et al., 2008, for a review). A previous study has indicated that body ownership disappears approximately after more than 300-ms delays, whereas the sense of agency is maintained to some extent after approximately less than 500 ms delays (Ismail & Shimada, 2016). These findings indicate that participants probably did not feel ownership of the virtual-hand avatar when there was a 440-ms delay, although they might have felt a sense of agency for the hand avatar. It is known that people feel body ownership of a rubber hand or a virtual-hand avatar moving concurrently with their own hand if the rubber hand or the virtual-hand avatar is placed near their own hand such that it is within PPS of their hand (Lloyd, 2007; see Blanke et al., 2015, for a review). The spatial properties of PPS determine body ownership’s spatial limits; however, PPS and body ownership do not have identical spatial mapping. Many previous studies have reported that hand-held, stick-like tools that are not considered to induce body ownership, can extend the hand-centered PPS to the tooltip (e.g., Farnè et al., 2005; Guterstam et al., 2018; Iriki et al., 1996; Maravita & Iriki, 2004; Serino et al., 2007). Therefore, spatial limits of PPS might depend more on how people can control their body or tools than the ownership of the body or tools. As a result, it is not problematic whether this study did not induce body ownership of the virtual-hand avatar. The delay in the virtual-hand avatar’s movement was consistent throughout the experiment, and participants could easily understand how they could control the virtual-hand avatar.
We showed that adaptation to a delay in body movement caused an extension of PPS. Many studies have shown that PPS extends under different situations, including both defensive and nondefensive situations, and has considered mechanisms behind PPS extension. These findings on PPS extensions and their functions can partially contribute to explain the mechanisms underlying the current findings. Magosso et al. (2010) developed a neural network model mimicking the human multisensory representation system representing PPS’s plasticity. This model includes feedforward and feedback interactions between unimodal (visual and tactile) and multisensory areas of the ventral premotor cortex (vPMC). Moreover, the model generated the hypothesis that nearly identical PPS extensions will be observed with or without tools. Serino et al. (2015) tested this hypothesis. They demonstrated that PPS extended without tool use after synchronous audio-tactile training and suggested that visual or auditory stimuli presented in distant areas and tactile stimuli delivered to the hand could be integrated by the Hebbian rule through synchronous audio-tactile or visuo-tactile training. These studies suggest that repeated, simultaneous, audio-tactile/visuo-tactile stimulation increases the synaptic efficacy between multisensory and unisensory neuronal responses to visual and auditory stimuli. This view, which presupposes highly established neural mechanisms in the brain, however, cannot explain the present results, because there was no synchronous visuo-tactile stimulation in this study. Many other studies have shown that PPS extends without synchronous audio-tactile/visuo-tactile training. Bassolino et al. (2010) demonstrated that using a computer mouse could extend PPS toward a display showing a cursor that moved synchronously with a mouse if participants often used computers in daily life. However, these participants did not experience audio-tactile/visuo-tactile training before the multisensory facilitation task. Several other studies have shown that PPS can be extended by walking on a treadmill or a foot-sole vibrator generating walking-like sensations in the absence of audio-tactile/visuo-tactile training (Amemiya et al., 2019; Noel et al., 2015). These findings suggest that extensions of auditory (or visual) receptive fields of multisensory neurons depend on knowledge of the spatio-temporal relationship between people and external stimuli (i.e., when and where people can interact, even not physically, with external stimuli). Synchronous audio-tactile/visuo-tactile training makes people understand the concurrence of events between distant positions where visual stimuli are presented and a body part where tactile stimuli are delivered. It does not involve physical interaction, but the visual stimuli in the distant area become a reference of the tactile stimulation on the body part, and vice versa. Walking on a treadmill extends PPS based on when people are expected to collide with visual or auditory stimuli. The multisensory neurons' receptive fields are determined by synchronous multisensory stimulation and the spatio-temporal relationship between people’s bodies (or body parts) and external stimuli. This plasticity helps prepare appropriate defensive or goal-directed actions before colliding with external objects. This perspective is supported by Fogassi et al. (1996), which showed that the visual receptive field sizes of monkey’s multisensory neurons are modulated according to the velocity of approaching visual stimuli. In the case of the present study, multisensory neurons anchored to the hand must integrate tactile (and sensorimotor) information around the hand and visual information about external stimuli positioned in more distant areas than usual when there is a delay in the hand movements to conduct appropriate actions on external stimuli, which resulted in the PPS extension. From this perspective, defensive and nondefensive functions of PPS might be supported by a common system that includes the spatio-temporal relationship between people’s bodies (or body parts) and external stimuli (see Bufacchi & Iannetti, 2018).
In sum, this study demonstrated that PPS extends when body movements are delayed, which is a finding that has substantial implications for the multisensory nature of the defensive PPS. On the other hand, many studies have shown that multisensory PPS extends in nondefensive situations (e.g., Bassolino et al., 2010). Therefore, the facilitation of reactions to tactile stimuli caused by visual or auditory stimuli near the body is probably related to both defensive and nondefensive functions of PPS. Although de Vignemont and Iannetti (2015) suggested that there is an apparent dissociation between defensive and nondefensive action, the findings of visuo-tactile and audio-tactile facilitation in defensive and nondefensive contexts might be evidence of common systems and interactions between defensive and nondefensive PPS (see Hunley & Lourenco, 2018, about this issue).
References
Amemiya, T., Ikei, Y., & Kitazaki, M. (2019). Remapping peripersonal space by using foot-sole vibrations without any body movement. Psychological Science, 30(10), 1522–1532. https://doi.org/10.1177/0956797619869337
Bassolino, M., Serino, A., Ubaldi, S., & Làdavas, E. (2010). Everyday use of the computer mouse extends peripersonal space representation. Neuropsychologia, 48(3), 803–811. https://doi.org/10.1016/j.neuropsychologia.2009.11.009
Blanke, O., Slater, M., & Serino, A. (2015). Behavioral, neural, and computational principles of bodily self-consciousness. Neuron, 88(1), 145–166. https://doi.org/10.1016/j.neuron.2015.09.029
Bufacchi, R. J., & Iannetti, G. D. (2018). An action field theory of peripersonal space. Trends in Cognitive Sciences, 22(12), 1076–1090. https://doi.org/10.1016/j.tics.2018.09.004
Cardinali, L., Brozzoli, C., & Farnè, A. (2009). Peripersonal space and body schema: Two labels for the same concept? Brain Topography, 21(3/4), 252–260. https://doi.org/10.1007/s10548-009-0092-7
de Vignemont, F., & Iannetti, G. D. (2015). How many peripersonal spaces? Neuropsychologia, 70, 327–334. https://doi.org/10.1016/j.neuropsychologia.2014.11.018
Farnè, A., Iriki, A., & Làdavas, E. (2005). Shaping multisensory action-space with tools: Evidence from patients with cross-modal extinction (Special issue). Neuropsychologia, 43(2), 238–248. https://doi.org/10.1016/j.neuropsychologia.2004.11.010
Fogassi, L., Gallese, V., Fadiga, L., Luppino, G., Matelli, M., & Rizzolatti, G. (1996). Coding of peripersonal space in inferior premotor cortex (area F4). Journal of Neurophysiology, 76(1), 141–157. https://doi.org/10.1152/jn.1996.76.1.141
Graziano, M. S. A., & Cooke, D. F. (2006). Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia, 44(13), 2621–2635. https://doi.org/10.1016/j.neuropsychologia.2005.09.011
Guterstam, A., Szczotka, J., Zeberg, H., & Ehrsson, H. H. (2018). Supplemental material for tool use changes the spatial extension of the magnetic touch illusion. Journal of Experimental Psychology: General, 147(2), 298–303. https://doi.org/10.1037/xge0000390.supp
Hunley, S. B., & Lourenco, S. F. (2018). What is peripersonal space? An examination of unresolved empirical issues and emerging findings. Wiley Interdisciplinary Reviews: Cognitive Science, 9(6), 1–17. https://doi.org/10.1002/wcs.1472
Iriki, A., Tanaka, M., & Iwamura, Y. (1996). Coding of modified body schema during tool use by macaque postcentral neurones. NeuroReport, 7(14), 2325–2330. https://doi.org/10.1097/00001756-199610020-00010
Ismail, M. A. F., & Shimada, S. (2016). “Robot” hand illusion under delayed visual feedback: Relationship between the senses of ownership and agency. PLOS ONE, 11(7), 1–9. https://doi.org/10.1371/journal.pone.0159619
Lloyd, D. (2007). Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition, 64(1), 104–109. https://doi.org/10.1016/j.bandc.2006.09.013
Lourenco, S. F., Longo, M. R., & Pathman, T. (2011). Near space and its relation to claustrophobic fear. Cognition, 119(3), 448–453. https://doi.org/10.1016/j.cognition.2011.02.009
Magosso, E., Ursino, M., di Pellegrino, G., Làdavas, E., & Serino, A. (2010). Neural bases of peri-hand space plasticity through tool-use: Insights from a combined computational-experimental approach. Neuropsychologia, 48(3), 812–830. https://doi.org/10.1016/j.neuropsychologia.2009.09.037
Makin, T. R., Holmes, N. P., & Ehrsson, H. H. (2008). On the other hand: Dummy hands and peripersonal space. Behavioural Brain Research, 191(1), 1–10. https://doi.org/10.1016/j.bbr.2008.02.041
Maravita, A., & Iriki, A. (2004). Tools for the body (schema). Trends in Cognitive Sciences, 8(2), 79–86. https://doi.org/10.1016/j.tics.2003.12.008
Mine, D., & Yokosawa, K. (2021a). Disconnected hand avatar can be integrated into the peripersonal space. Experimental Brain Research, 239(1), 237–244. https://doi.org/10.1007/s00221-020-05971-z
Mine, D., & Yokosawa, K. (2021b). Remote hand: Hand-centered peripersonal space transfers to a disconnected hand avatar. Attention, Perception, & Psychophysics, 83, 3250–3258. https://doi.org/10.3758/s13414-021-02320-2
Noel, J. P., Grivaz, P., Marmaroli, P., Lissek, H., Blanke, O., & Serino, A. (2015). Full body action remapping of peripersonal space: The case of walking. Neuropsychologia, 70, 375–384. https://doi.org/10.1016/j.neuropsychologia.2014.08.030
Poliakoff, E., Miles, E., Li, X., & Blanchette, I. (2007). The effect of visual threat on spatial attention to touch. Cognition, 102(3), 405–414. https://doi.org/10.1016/j.cognition.2006.01.006
Rizzolatti, G., Fadiga, L., Fogassi, L., & Gallese, V. (1997). The space around us. Science, 277(5323), 190–191. https://doi.org/10.1126/science.277.5323.190
Sambo, C. F., & Iannetti, G. D. (2013). Better safe than sorry? The safety margin surrounding the body is increased by anxiety. Journal of Neuroscience, 33(35), 14225–14230. https://doi.org/10.1523/JNEUROSCI.0706-13.2013
Sambo, C. F., Liang, M., Cruccu, G., & Iannetti, G. D. (2012). Defensive peripersonal space: The blink reflex evoked by hand stimulation is increased when the hand is near the face. Journal of Neurophysiology, 107(3), 880–889. https://doi.org/10.1152/jn.00731.2011
Schiff, W. (1965). Perception of impending collision: A study of visually directed avoidant behavior. Psychological Monographs: General and Applied, 79(11), 1–26.
Serino, A. (2019). Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neuroscience and Biobehavioral Reviews, 99, 138–159. https://doi.org/10.1016/j.neubiorev.2019.01.016
Serino, A., Bassolino, M., Farnè, A., & Làdavas, E. (2007). Extended multisensory space in blind cane users. Psychological Science, 18(7), 642–648. https://doi.org/10.1111/j.1467-9280.2007.01952.x
Serino, A., Canzoneri, E., Marzolla, M., di Pellegrino, G., & Magosso, E. (2015). Extending peripersonal space representation without tool-use: Evidence from a combined behavioral-computational approach. Frontiers in Behavioral Neuroscience, 9, 1–14. https://doi.org/10.3389/fnbeh.2015.00004
Serino, A., Noel, J. P., Mange, R., Canzoneri, E., Pellencin, E., Ruiz, J. B., Bernascone, F., Blanke, O., & Herbelin, B. (2017). Peripersonal space: An index of multisensory body-environment interactions in real, virtual, and mixed realities. Frontiers in ICT, 4, 1–12. https://doi.org/10.3389/fict.2017.00031
Taffou, M., & Viaud-Delmon, I. (2014). Cynophobic fear adaptively extends peri-personal space. Frontiers in Psychiatry, 5, 3–9. https://doi.org/10.3389/fpsyt.2014.00122
Vagnoni, E., Lourenco, S. F., & Longo, M. R. (2012). Threat modulates perception of looming visual stimuli. Current Biology, 22(19), R826–R827. https://doi.org/10.1016/j.cub.2012.07.053
Vagnoni, E., Lourenco, S. F., & Longo, M. R. (2015). Threat modulates neural responses to looming visual stimuli. European Journal of Neuroscience, 42(5), 2190–2202. https://doi.org/10.1111/ejn.12998
Zadra, J. R., & Clore, G. L. (2011). Emotion and perception: The role of affective information. Wiley Interdisciplinary Reviews: Cognitive Science, 2(6), 676–685. https://doi.org/10.1002/wcs.147
Acknowledgments
This work was supported by Japan Society for the Promotion of Science [Grant Numbers: 19H01490]. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: D.M., K.Y. Performed the experiment: D.M. Analyzed the data: D.M. Wrote the paper: D.M., K.Y.
Corresponding author
Additional information
Open practices statements
The data that support the findings of this study are not openly available and are available from the corresponding author upon reasonable request. None of the materials for the experiments reported here is available, and none of the experiments was preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mine, D., Yokosawa, K. Adaptation to delayed visual feedback of the body movement extends multisensory peripersonal space. Atten Percept Psychophys 84, 576–582 (2022). https://doi.org/10.3758/s13414-021-02425-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-021-02425-8