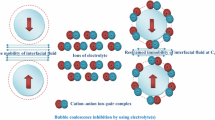
Abstract
An experiment on laser diagnostics of gas bubbles moving in aqueous solutions of different electrolytes has revealed that efficient suppression of gas bubble coalescence (collapse) occurs at certain concentrations of cation−anion pairs. It is experimentally proven that there is an optimal ion composition at which the coalescence is suppressed in a wide range of velocities of gas particle flow through a column with liquid. It is shown that the threshold ion concentrations corresponding to the onset of coalescence suppression decrease with an increase in the external pressure in the liquid. The results obtained can be used in oil production technologies to solve the problem of burning associated oil gas in torches.
Similar content being viewed by others
References
V.V. Sazhin, I. Seldinas, and V.B. Sazhin, “Russia’s Hard-to-Recover Reserves and Heavy Oil,” J. Adv. Chem. Chem. Tech. 22(12(92)), 56 (2008) [in Russian].
www.expert.ru/ural/2014/37/trudnoizvlekaemyijdohod/
www.burneft.ru/archive/issues/2012-08/7
www.finmarket.ru/main/article/3469367
www.wwf.ru/resources/publ/book/837
izvestia.ru/news/568209
Yu. G. Frolov, Course of Colloidal Chemistry (Khimiya, Moscow, 1982) [in Russian].
V.S.J. Craig, B.W. Ninham, and R.M. Pashley, “Effect of Electrolytes on Bubble Coalescence,” Nature. 364, 317 (1993).
B.W. Ninham and R.M. Pashley, “The Effect of Electrolytes on Bubble Coalescence in Water,” J. Phys. Chem. 97, 10192 (1993).
R.M. Pashley and V.S.J. Craig, “Effects of Electrolytes on Bubble Coalescence,” Langmuir. 13(17), 4772 (1997).
V.S.J. Craig, “Bubble Coalescence and Specific-Ion Effects,” Current Opinion in Colloid & Interface Science. 9(1-2), 178 (2004).
C.L. Henry, C.N. Dalton, L. Scruton, and V.S.J. Craig, “Ion-Specific Coalescence of Bubbles in Mixed Electrolyte Solutions,” J. Phys. Chem. C. 111(2), 1015 (2007).
C.L. Henry and V.S.J. Craig, “The Link between Ion Specific Bubble Coalescence and Hofmeister Effects Is the Partitioning of Ions within the Interface,” Langmuir. 26(9), 6478 (2010).
C.L. Henry and V.S.J. Craig, “Ion-Specific Influence of Electrolytes on Bubble Coalescence in Nonaqueous Solvents,” Langmuir. 24(15), 7979 (2008).
G. Liu, Y. Hou, G. Zhang, and V.S.J. Craig, “Inhibition of Bubble Coalescence by Electrolytes in Binary Mixtures of Dimethyl Sulfoxide and Propylene Carbonate,” Langmuir. 25(18), 10495 (2009).
C.L. Henry and V.S.J. Craig, “Inhibition of Bubble Coalescence by Osmolytes: Sucrose, Other Sugars, and Urea,” Langmuir. 25(19), 11406 (2009).
L.D. Landau and E.M. Lifshitz, Course of Theoretical Physics. Vol.6: Hydrodynamics (Pergamon, Oxford, 1984).
E.J. Verwey and J.T.G. Overbeck, Theory of the Stability of Lyophobic Colloids (Elsevier, Amsterdam, 1948).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bunkin, N.F., Drozdov, A.N., Drozdov, N.A. et al. Suppression of the coalescence of gas bubbles in aqueous electrolyte solutions: dependence on the external pressure and velocity of gas flow through a column with liquid. Phys. Wave Phen. 25, 219–224 (2017). https://doi.org/10.3103/S1541308X17030098
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1541308X17030098