Abstract
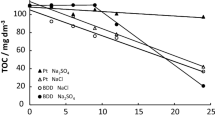
The paper reports the oxidative decolorization of the direct blue 14 (DB-14) dye in an aqueous phase via an ecologically friendly electrochemical advanced oxidation process. The investigation of several experimental conditions such as the solution pH, initial concentrations of the oxidant, the dye, and current density was carried out in a single-compartment electrolytic cell with the use of an iron sheet as anodic material, the graphite plate being the cathodic material. The optimal operating conditions were found to be as follows: (DB-14)0—500 mg/L; (H2O2)0—35 mM concentration; pH 3 using 0.1 M Na2SO4 salt as supportive electrolyte at room temperature. It has been observed that the dissolved iron (Fe2+) content from the anode was continuously increasing in aqueous media on passing a constant current to it. Initially, it was 1.58 mM, then 4.46 mM (after 30 min of reaction time), and 5.35 mM of dissolved Fe2+ for 60 min of the reaction time. The study of kinetics for the decolorization of DB-14 shows that the degradation kinetics follows the second order, with the reaction rate being constant: k2—0.5 × 10–4 mgL–1 min–1. Electrochemical oxidation of DB-14 reduces the chemical oxygen demand of the colored solution. The unit energy demand, expressed in kWh/kg of the chemical oxygen demand reduced, for optimum experimental parameters have also been determined.





Similar content being viewed by others
REFERENCES
Carvalho, S.S.F. and Carvalho, N.M.F., J. Environ. Manage., 2017, vol. 187, pp. 82–88.
Dang, T.D., Banerjee, A.N., Tran, Q.T., and Roy, S., J. Phys. Chem. Solids, 2016, vol. 98, pp. 50–58.
Alvi, M.A., Al-ghamdi, A.A., and Shaheerakhtar, M., Mater. Lett., 2017, vol. 204, pp. 12–15.
Lee, W.P.C., Wong, F.H., Attenborough, N.K., Kong, X.Y., et al., J. Environ. Manage., 2017, vol. 197, pp. 63–69.
Singh, R.L., Singh, P.K., and Singh, R.P., Int. Biodeterior. Biodegrad., 2015, vol. 104, pp. 21–31.
Leite, L.S., Maselli, B.S., Umbuzeiro, G.A., and Nogueira, R.F.P., Chemosphere, 2016, vol. 148, pp. 511–517.
Kalal, S., Chanderia, K., Meghwal, K., Chouhan, N.P.S., et al., Indian J. Chem. Technol., 2015, vol. 22, pp. 148–154.
Velmurugan, R., Krishnakumar, B., Kumar, R., and Swaminathan, M., Arab. J. Chem., 2012, vol. 5, pp. 447–452.
Nadaroglu, H., Cicek, S., and Alayli, A., Spectrochim. Acta, Part A, 2016, vol. 172, pp. 2–8.
Rao, A.N.S. and Venkatarangaiah, V.T., J. Electrochem. Sci. Eng., 2013, vol. 3, pp. 167–184.
Dimoglo, A., Akbulut, H.Y., Cihan, F., and Karpuzcu, M., Clean Technol. Environ. Policy, 2004, vol. 6, pp. 288–295.
Kayan, B., Demirel, M., and Gizir, A.M., J. Hazard. Mater., 2010, vol. 177, pp. 95–102.
Bensalah, N., Alfaro, M.A.Q., and Martinez-Huitle, C.A., Chem. Eng. J., 2009, vol. 149, pp. 348–352.
Kourdali, S., Badis, A., and Boucherit, A., Ecotoxicol. Environ. Saf., 2014, vol. 110, pp. 110–120.
Olvera-Vargas, H., Oturan, N., and Oturan, M.A., Proc. 3rd Int. Conf. on Development, Energy, Environment, Economics, Athens: World Sci. Eng. Acad. Soc., 2012, pp. 99–104.
Lucas, M.S., Dyes Pigm., 2006, vol. 71, pp. 236–244.
Ghoneim, M.M., El-Desoky, H.S., and Zidan, N.M., Desalination, 2011, vol. 274, pp. 22–30.
Sahinkaya, S., J. Ind. Eng. Chem., 2013, vol. 19, pp. 601–605.
Fernades Rego, F.E., Sales Solano, A.M., da Costa Soares, I.C., da Silva, D.R., et al., J. Environ. Chem. Eng., 2014, vol. 2, pp. 875–880.
Martínez, S.S. and Uribe, E.V., Ultrason. Sonochem., 2012, vol. 19, pp. 174–178.
Ozcan, A. and Gencten, M., Chemosphere, 2016, vol. 146, pp. 245–252.
Khataee, A.R., Vatanpour, V., Amani Ghadim, A.R., J. Hazard. Mater., 2009, vol. 161, pp. 1225–1233.
El-sayed, G.O., Teleb, S.M., and Gouda, H.M., J. Basic Environ. Sci., 2017, vol. 4, pp. 18–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare to have no conflict of interest.
About this article
Cite this article
Damodhar Ghime, Prabir Ghosh Electrochemical Oxidation of Direct Blue 14 in Aqueous Phase: Experimental and Kinetic Studies. Surf. Engin. Appl.Electrochem. 56, 282–288 (2020). https://doi.org/10.3103/S1068375520030047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375520030047