Abstract
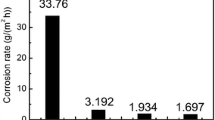
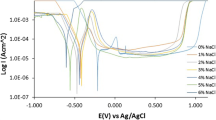
The susceptibility of AISI (American iron and steel institute) 316L austenitic stainless steel alloy to pitting corrosion was assessed in 3.5% chloride solutions containing various concentration of thiosulfate ions, a main sulfide oxidant product, spanning across values of 0.001, 0.005, 0.01, 0.05 and 0.1 M, at temperatures of 23, 50 and 80°C. The potentiodynamic scan results indicated that low thiosulfate concentrations promote the chloride attack and the aggressiveness of thiosulfate species depends on the chloride to thiosulfate ratio and the test temperature. Increasing temperature apparently promotes the ionic activity of Cl– and S2O32– The thiosulfate to chloride ratio plays an essential role in pitting the intensity of the AISI 316L stainless steel alloy and was found to be dependent on the test temperature.
Similar content being viewed by others
References
Silva, C., Farias, J., and de Sant’Ana, H., Mater. Des., 2009, vol. 30, pp. 1581–1587.
Olsson, J., Desalination, 2005, vol. 183, pp. 217–225.
Oldfield, J. and Todd, B., Desalination, 1999, vol. 124, pp. 75–84.
Silva, C., de Miranda, H., de Sant’Ana, H., and Farias, J., Mater. Charact., 2009, vol. 60, pp. 346–352.
Meng, G., Li, Y., Shao, Y., Zhang, T., Wang, Y., and Wang, F., J. Mater. Sci. Techol., 2014, vol. 30, pp. 253–258.
Ezuber, H., Mater. Des., 2014, vol. 59, pp. 330–343.
Newman, R.C., Bandy, R., and Roberge, R., Corrosion, 1983, vol. 39, pp. 386–390.
Almorshad, A. and Jamal, D., J. Appl. Electrochem., 2004, vol. 34, pp. 67–70.
Laycock, N., Corrosion, 1999, vol. 55, pp. 590–595.
Newman, R.C., Wong, W.P., Ezuber, H.M., and Garner, A., Corrosion, 1989, vol. 45, pp. 282–287.
Roberge, P., Wang, S., and Roberge, R., Corrosion, 1999, vol. 57, pp. 733–737.
Ezuber, H.M. and Newman, R.C., Proc. European Corrosion Congr. EUROCORR’97, September 22–25, 1997, Trondheim, 1997, vol. 1, pp. 401–407.
Newman, R.C., Corrosion, 1985, vol. 41, pp. 450–453.
Garner, A., Corrosion, 1985, vol. 41, pp. 587–591.
Betts, A.J. and Newman, R.C., Corros. Sci., 1985, vol. 25, pp. 1551–1555.
Albiache, A. and Marcus, P., Corros. Sci., 1992, vol. 33, pp. 261–269.
Zhang, J. and Millero, F., Geochim. Cosmochim. Acta, 1993, vol. 57, pp. 1705–1718.
Newman, R.C., Wong, W.P., and Garner, A., Corrosion, 1986, vol. 42, pp. 489–491.
Horowtiz, H.H., Corros. Sci., 1983, vol. 23, pp. 353–362.
Ramana, K.V.S., Anita, T., Mandal, S., Kaliappan, S., Shaikh, H., Sivaprasad, P.V., Dayal, R.K., and Khatak, H.S., Mater. Des., 2009, vol. 30, pp. 3770–3775.
Laycock, N. and Newman, R.C., Corros. Sci., 1998, vol. 40, pp. 887–902.
Semino, C., Pedeferri, P., Burstein, G., and Hoar, T., Corros. Sci., 1970, vol. 19, pp. 1069–1078.
Souza, E., Rossitti, S., and Rollo, J., Mater. Charact., 2010, vol. 61, pp. 240–244.
Laitinen, T., Corros. Sci., 2000, vol. 42, pp. 421–441.
Ezuber, H. and Newman, R.C., Proc. III Symp. on Critical Factors in Localized Corrosion, ECS Proceedings Series, Frankel, G. and Newman, R.C., Eds., Pennington, NJ: Electrochem. Soc., 1992, vol. 92/9, pp. 120–133.
Ezuber, H., Betts, A.J., and Newman, R.C., Mater. Sci. Forum, 1989, vols. 44–45, pp. 247–258.
Marcus, P. and Protopopoff, E., Corros. Sci., 1997, vol. 39, pp. 1741–1752.
Ezuber, H., J. ASTM Int., 2005, vol. 2. doi 10.1520/JAI12866
Newman, R.C., Isaacs, H., and Alman, B., Corrosion, 1982, vol. 38, pp. 261–265.
Park, J.O., Verhoff, M., and Alkire, A., Electrochim. Acta, 1997, vol. 42, pp. 3281–3291.
Ezuber, H., J. ASTM Int., 2005, vol. 2, pp. 96–106.
Ezuber, H., J. ASTM Int., 2009, vol. 6. doi 10.1520/JAI101709
Duret-Thual, C., Costa, D., Yang, W.P., and Marcus, P., Corros. Sci., 1997, vol. 39, pp. 913–933.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Ezuber, H., Alshater, A. & Abulhasan, M. Role of thiosulfate in susceptibility of AISI 316L austenitic stainless steels to pitting corrosion in 3.5% sodium chloride solutions. Surf. Engin. Appl.Electrochem. 53, 493–500 (2017). https://doi.org/10.3103/S1068375517050052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375517050052