Abstract
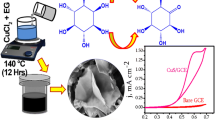
The electrochemical responses of ascorbic acid at the Cu-sulfide modified glassy carbon electrode have been analyzed by adjusting the scan rate, ascorbic acid concentration and species of the electrolytes. Two anodic cyclic voltammogram peaks (cvp1, cvp2) are located at +0.56 V and +0.33 V, and two cathodic cyclic voltammogram peaks (cvp1′, cvp2′) are located at +0.10 V and–0.40 V, respectively. The intensities of the cyclic voltammogram peaks increase linearly with the increase of the ascorbic acid concentration in a range of 0.0005–2 mM and scan rate ranging from 25 to 200 mV s–1. A Cu-sulfide modified glassy carbon electrode shows good determination ability for ascorbic acid in neutral solutions with the detection limit of 0.18 μM and 0.12 μM for cvp1 and cvp2, respectively, at a signal-to-noise ratio of 3. Cu sulfide modified glassy carbon electrode shows good reproducibility and stability.
Similar content being viewed by others
References
Xia, C. and Ning, W., Analyst, 2011, vol. 136, pp. 288–292.
Li, Y. and Zhan, S.H., J. Dispersion Sci. Technol., 2008, vol. 29, pp. 1421–1425.
Arrigoni, O. and Tullio, C.D., Biochim. Biophys. Acta, Gen. Subj., 2002, vol. 1569, pp. 1–9.
Barberis, A., Bazzu, G., Galia, G., Puggioni, G.M., et al., Anal. Chem., 2010, vol. 82, pp. 5134–5140.
Guth, U., Voau, W., and Zosel, J., Meas. Sci. Technol., 2009, vol. 20, p. 042002.
Liu, A.H., Biosens. Bioelectron., 2008, vol. 24, pp. 167–177.
Zhang, M.N., Liu, K., Xiang, L., Lin, Y.Q., et al., Anal. Chem., 2007, vol. 79, pp. 6559–6565.
Donf, S. and Wang, J., Electroanalysis, 1989, vol. 1, pp. 99–106.
Zhang, J., Deng, P.H., Feng, Y.L., Kuang, Y.F., et al., Microchim. Acta, 2004, vol. 147, 279–282.
Keeley, G.P., O’Neill, A., MeEvoy, N., Peltekis, N., and Coleman, J.N., J. Mater. Chem., 2010, vol. 20, pp. 7864–7869.
Florou, A.B., Prodromidis, M.I., Karayannis, M.I., and Tzouwara-Karayanni, S.M., Anal. Chim. Acta, 2000, vol. 409, pp. 113–121.
Pei, L.Z., Cai, Z.Y., Xie, Y.K., Pei, Y.Q., Fan, C.G., and Fu, D.G., J. Electrochem. Soc., 2012, vol. 159, pp. G107–G111.
Erokhina, S., Erokhin, V., Nicolini, C., Sbrana, F., Ricci, D., and Zittl, E., Langmuir, 2003, vol. 19, pp. 766–771.
Rejinen, L., Meester, B., Goossens, A., and Schoonman, J., Chem. Vap. Deposition, 2003, vol. 9, pp. 15–20.
Chung, J.S. and Sohn, H.J., J. Power Sources, 2002, vol. 108, pp. 226–231.
Pei, L.Z., Wang, J.F., Yang, L.J., Wang, S.B., Dong, Y.P., Fan, C.G., and Zhang, Q.F., Mater. Charact., 2011, vol. 62, pp. 354–359.
Pei, L.Z., Xie, Y.K., Cai, Z.Y., Yang, Y., Pei, Y.Q., Fan, C.G., and Fu, D.G., J. Electrochem. Soc., 2012, vol. 159, pp. K55–K60.
Pei, L.Z., Lin, N., Wei, T., Liu, H.D, and Yu, H.Y., J. Mater. Chem. A, 2015, vol. 3, pp. 2690–2700.
Davis, J., Moorcroft, M., Wilkins, S.J., Compton, R.G., and Cardosi, M.F., Analyst, 2000, vol. 125, pp. 737–742.
Özcan, L., Sahin, M., and Sahin, Y., Sensors, 2008, vol. 8, pp. 5792–5805.
Kumar, S.A., Lo, P.H., and Chen, S.M., Biosens. Bioelectron., 2008, vol. 24, pp. 518–524.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Pei, L.Z., Lin, N., Wei, T. et al. Electrochemical determination of ascorbic acid using Cu sulfide modified glassy carbon electrode. Surf. Engin. Appl.Electrochem. 52, 565–571 (2016). https://doi.org/10.3103/S1068375516060120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375516060120