Abstract
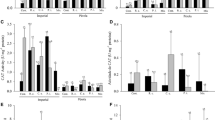
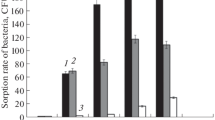
The effect of inoculation with endotrophic micromycete Cylindrocarpon magnusianum on the physiological and biochemical parameters of test tomato plants when exposed to heavy metal salts was studied. The experimental scheme included inoculation with a fungal culture (control population) and with populations of this fungus that were previously adapted to this stress factor. The inoculated plants were then grown under control conditions and on substrates with different concentrations of heavy metal salts (zinc, copper, lead, and chromium). No stimulating effect that increases the resistance of plants to heavy metal salts after inoculation of plants by the control population of the fungus C. magnusianum was detected. When using nonbiogenic chemical elements, adaptive plant responses associated with the content of photosynthetic pigments in leaves and the formation of plant biomass were significantly expressed when plants were inoculated with adapted fungal populations of C. magnusianum and when they were further cultivated on substrates introduced with chromium and lead salts. Under these conditions, the fungal infection in plant roots was more intense compared to the use of the control population of the fungus. These facts indicate the more efficient partnership of the fungus C. magnusianum and the root system of plants under conditions that are extreme for plant life.



Similar content being viewed by others
REFERENCES
Wilkinson, D.M., At cross purposes, Nature, 2001, vol. 412, p. 485. https://doi.org/10.1038/35087676
Yurkov, A.P., Kryukov, A.A., Gorbunova, A.O., Kozhemyakov, A.P., Stepanova, G.V., Machs, E.M., Radionov, A.V., and Shishova, M.F., Molecular genetic identification of arbuscular mycorrhiza fungi, Ekol. Genet., 2018, no. 16, pp. 11–23. https://doi.org/10.17816/ecogen16211-23
Ijdo, M., Cranenbrouck, S., and Declerck, S., Methods for large-scale production of AM fungi: Past, present and future, Mycorrhiza, 2011, vol. 21, pp. 1–16. https://doi.org/10.12691/ijebb-4-1-1
Rodriguez, R.J., White, J.F., Arnold, A.E., and Redman, R.S., Fungal endophytes: Diversity and functional roles, New Phytol., 2009, vol. 182, pp. 314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
El-Samad, H.M. and El-Hakeem, K.N.S., Strategy role of mycorrhiza inoculation on osmotic pressure, chemical constituents and growth yield of maize plant gown under drought stress, Am. J. Plant Sci., 2019, vol. 10, no. 6, pp. 1102–1120. https://doi.org/10.4236/ajps.2019.106080
Bilal, S., Shahzad, R., Imran, M., Jan, R., Min, K., and Lee, I.-J., Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress, Ind. Crops Prod., 2020, vol. 143, pp. 1–10. https://doi.org/10.1016/j.indcrop.2019.111931
Dabral, S., Yashaswee, Varma, A., Choudhary, D.K., Bahuguna, R.N., and Nath, M., Biopriming with Piriformospora indica ameliorates cadmium stress in rice by lowering oxidative stress and cell death in root cells, Ecotoxicol. Environ. Saf., 2019, vol. 186, pp. 1–12. https://doi.org/10.1016/j.ecoenv.2019.109741
Ikram, M., Ali, I.N., Jan, G., Jan, F.G., Rahman, I.U., Iqbal, A., and Hamayun, M., IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils, PLoS One, vol. 13, no. 11, e0208150. https://doi.org/10.1371/journal.pone.0208150
Hou, L., Yu, J., Zhao, L., and He, X., Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress, Front. Microbiol., 2020, vol. 10, pp. 1–17. https://doi.org/10.3389/fmicb.2019.03061
Bilal, S., Shahzad, R., Khan, A.L., Al-Harrasi, A., Kim, C.K., and Lee, I.-J., Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stressresponsive proteins modulation, J. Hazard. Mater., 2019, vol. 379, p. 120824. https://doi.org/10.1016/j.jhazmat.2019.120824
Li, X., Zhang, X., Wang, X., Yang, X., and Cui, Z., Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum, Chemosphere, 2019, vol. 224, pp. 716–725. https://doi.org/10.1016/j.chemosphere.2019.02.184
Sharma, V.K., Li, X., Wu, G., Bai, W., Parmar, S., White, Jr, J.F., and Li, H., Endophytic community of Pb-Zn hyperaccumulator Arabis alpina and its role in host plants metal tolerance, Plant Soil, 2019, vol. 437, pp. 397–411. https://doi.org/10.1007/s11104-019-03988-0
Ali, A., Bilal, S., Khan, A.L., Mabood, F., Al-Harrasi, A., and Lee, I.-J., Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress, J. Plant Interact., 2019, vol. 14, no. 1, pp. 303–313. https://doi.org/10.1080/17429145.2019.1633428
Boyette, C.D., Hoagland, R.E., and Stetina, K.C., Efficacy improvement of a bioherbicidal fungus using a formulation-based approach, Am. J. Plant Sci., 2016, vol. 7, no. 16, pp. 2349–2358. https://doi.org/10.4236/ajps.2016.716206
Boyette, C.D., Hoagland, R.E., and Stetina, K.C., Hot water treatment enhances the bioherbicidal efficacy of a fungus, Am. J. Plant Sci., 2018, vol. 9, no. 10, pp. 2063–2076. https://doi.org/10.4236/ajps.2018.910150
Meepagala, K.M., Clausen, B.M., Johnson, R.D., Wedge, D.E., and Duke, S.O., A phytotoxic and antifungal metabolite (pyrichalasin H) from a fungus infecting Brachiaria eruciformis (signal grass), J. Agric. Chem. Environ., 2019, vol. 8, no. 3, pp. 115–128. https://doi.org/10.4236/jacen.2019.83010
Sobowale, A.A., Probable effects of dual inoculation of maize (Zea mays) stem with Fusarium verticillioides and certain trichoderma species on fumonisin content of maize seeds, Am. J. Plant Sci., 2019, vol. 10, no. 5, pp. 752–759. https://doi.org/10.4236/ajps.2019.105055
Sogonov, M.V. and Velikanov, L.L., Soil microfungi from alpine and subnival ecosystems of the Northwestern Caucasus, Mikol. Fitopatol., 2004, no. 38, pp. 50–58.
Amaral, D.R., Oliveira, D.F., Campos, V.P., de Carvalho, D.A., and Nunes, A.S., Effect of plant and fungous metabolites on Meloidogyne exigua, Cienc. Agrotecnol., 2009, no. 33, pp. 1861–1865. https://doi.org/10.1590/S1413-70542010000500021
Bukharina, I.L. and Islamova, N.A., Investigation of the limits of resistance of microscopic fungi and the formation of a collection of promising isolates, Mat. godichnoe sobranie obshchestva fiziologov rastenii Rossii “Signal’nye sistemy rastenii: Ot retseptora do otvetnoi reaktsii organizma” (Proc. Annual Meeting of the Society of Plant Physiologists of Russia Signal Systems of Plants: From Receptor to Response of the Organism), St. Petersburg, 2016, pp. 362–363.
Bukharina, I.L., Islamova, N.A., Zhavad, A.F., Lebedeva, M.A., and Shashov, L.O., Effect of Cylindrocarpon magnusianum inoculum on the formation of plant adaptive responses to stress factors, Agrar. Ross., 2019, no. 12, pp. 26–32.
Bukharina, I.L., Islamova, N.A., Zhavad, A.F., Abdullakh, M.R., Lebedeva, M.A., and Shashov, L.O., Features of the formation of metal resistance during inoculation of tomato with micromycete Cylindrocarpon magnusianum, Estestv. Tekh. Nauki, 2019, no. 10, pp. 105–112.
Bukharina, I., Franken, Ph., Kamasheva, A., Vedernikov, K., and Islamova, N., About the species composition of microscopic fungi in soils and woody plant roots in urban environment, Int. J. Adv. Biotechnol. Res., 2016, no. 7, pp. 1386–1394. http://www.bipublication.com.
Bukharina, I.L. and Islamova, N.A., RU Patent 2722206, 2020.
Shtark, O.Yu. and Labutova, N.M., Traditsionnye metody raboty s arbuskulyarno-mikoriznymi gribami (Traditional Methods of Working with Arbuscular-Mycorrhizal Fungi), St. Petersburg: VNIISKhM, 2014.
Funding
This work was supported by the Russian Foundation for Basic Research’s Aspirant grant, no. 19-31690003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Bukharina, I.L., Islamova, N.A. & Lebedeva, M.A. Effect of Inoculating the Root System of Plants with Endophyte Cylindrocarpon magnusianum on Plant Performance When Exposed to Heavy Metal Salts. Russ. Agricult. Sci. 47, 42–47 (2021). https://doi.org/10.3103/S1068367421010067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367421010067