Abstract
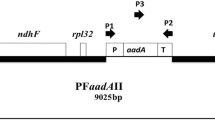
Transplastomic tobacco Nicotiana tabacum cv. Petit Havana plants were obtained by ballistic transformation. The modified expression cassette aadA au was used as a marker, which confers the resistance to streptomycin and spectinomycin and causes the golden color of the leaf when planted in the soil after selection on selective medium. Plants were transferred into soil and screened for the golden leaf color. Molecular analysis was performed to confirm the transplastomic nature of the plants. The differences in plastid ultrastructure of mesophyll cells between the leaves of tobacco plants of wild-type (with green leaf fragments) and plastids with altered pigmentation (old leaves of wild-type and yellow pieces of the leaf with transformed chloroplasts) were analyzed by electron microscopy. It was shown that the ultrastructural organization of plastids of transplastomic plants with golden color had distinct differences from the chloroplasts of plants in the control group by the number and arrangement of thylakoids, the number and sizes of starch grains and plastoglobules. Decreased number of thylakoids and starch grain inclusions was marked in the mesophyll cells on a background of their increased number in the stroma and grana. There were also significantly more light areas of the stroma in transplastomic plants, which was typical for nucleoid areas.
Similar content being viewed by others
References
Leister, D., Chloroplast research in the genomic age, Trends Genet., 2003, vol. 19, pp. 47–56.
Lopez-Juez, E. and Pyke, K.A., Plastids unleashed: their development and their integration in plant development, Int. J. Dev. Biol., 2005, vol. 49, pp. 557–577.
Pogson, B.J. and Albrecht, V., Genetic dissection of chloroplast biogenesis and development: an overview, Plant Physiol., 2011, vol. 155, pp. 1545–1551
Bendich A.J., Why do chloroplasts and mitochondria contain so many copies of their genome? BioEssays, 1987, vol. 6, pp. 279–282.
Maliga, P., Plastid transformation in higher plants, Annu. Rev. Plant Biol., 2004, vol. 55, pp. 289–313.
Tungsuchat-Huang, T., Sliminski, K.M., SinagawaGarcia, S.R., and Maliga, P., Visual spectinomycin resistance gene for facile identification of transplastomic sectors in tobacco leaves, Plant Mol. Biol., 2011, vol. 76, pp. 453–461.
Vera, A. and Sugiura, M., Chloroplast rRNA transcription from structurally different tandem promoters: an additional novel-type promoter, Curr. Genet., 1995, vol. 27, pp. 280–284.
Kuroda, H. and Maliga, P., Over-expression of the clpP 5′-UTR in chimeric context causes a mutant phenotype suggesting competition for a clpP-specific RNA maturation factor in tobacco chloroplasts, Plant Physiol., 2002, vol. 129, pp. 1600–1606.
Radugina, G.N., Yur’eva, N.O., and Danilova, S.A., Agrobaterium-related transformation of the plants, in Molekulyarno-geneticheskie i biokhmicheskie metody v sovremennoi biologii rastenii (Molecular-Genetic and Biochemical Methods Used in Modern Plant Biology), Kuznetsov, Vl.V., Kuznetsov, V.V., and Romanov, G.A., Eds., Moscow: Binom, 2011, pp. 5–26.
Danilova, S.A., Implementation of ballistic transformation for obtaining of transplastome tobacco plants, in Molekulyarno-geneticheskie i biokhmicheskie metody v sovremennoi biologii rastenii (Molecular-Genetic and Biochemical Methods Used in Modern Plant Biology), Kuznetsov, Vl.V., Kuznetsov, V.V., and Romanov, G.A., Eds., Moscow: Binom, 2011, pp. 26–36.
Belyaev, D.V., Southern blot analysis, in Molekulyarnogeneticheskie i biokhmicheskie metody v sovremennoi biologii rastenii (Molecular-Genetic and Biochemical Methods Used in Modern Plant Biology), Kuznetsov, Vl.V., Kuznetsov, V.V., and Romanov, G.A., Eds., Moscow: Binom, 2011, pp. 109–120.
Doyle, J.J. and Doyle, J.L., A rapid DNA isolation procedure for small quantities of fresh leaf tissue, Phytochem. Bull., 1987, vol. 19, pp. 11–15.
Reynolds, E.S., The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, pp. 208–212.
Baranova, E.N., Serenko, E.K., Balachnina, T.I., Kosobruhov, A.A., Kurenina, L.V., Gulevich, A.A., and Maisuryan, A.N., Activity of the photosynthetic apparatus and antioxidant enzymes in leaves of transgenic Solanum lycopersicum and Nicotiana tabacum plants, with FeSODn1 gene, Russ. Agric. Sci., 2010, vol. 36, pp. 242–249.
Myouga, F., Hosoda, Ch., Umezawa, T., Lizumi, H., Kuromori, T., Motohashi, R., Shono, Y., Nagata, N., Ikeuchi, M., and Shinozaki, K.A., Heterocomplex of iron superoxide dismutases defense chloroplast nucleoids against oxidative stress and is essential for chloroplast development, Plant Cell, 2008, vol. 20, pp. 3148–3162.
Serenko, E.K., Baranova, E.N., Balakhnina, T.I., Kurenina, L.V., Gulevich, A.A., Kosobruhov, A.A., Maysurian, A.N., and Polyakov, V.Yu., Structural organization of chloroplast of tomato plants Solanum lycopersicum transformed by Fe-containing superoxide dismutase, Biochem. (Moscow) Suppl. Ser. A: Membr. Cell Biol., 2011, vol. 5, pp. 177–184.
Jiang, Q., Ma, X.J., Gong, X.D., Zhang, J.H., Teng, S., Xu, J.L., Lin, D.Z., and Dong, Y.J., The rice OsDG2 encoding a glycine-rich protein is involved in the regulation of chloroplast development during early seedling stage, Plant Cell Rep., 2014, pp. 1–12.
Sakamoto, W., Uno, Y., Zhang, Q., Miura, E., and Yusuke, K., Sodmergen arrested differentiation of proplastids into chloroplasts in variegated leaves characterized by plastid ultrastructure and nucleoid morphology, Plant Cell Physiol., 2009, vol. 50, pp. 2069–2083.
Svab, Z. and Maliga, P., High-frequency plastid transformation in tobacco by selection for a chimeric and A gene, Proc. Natl. Acad. Sci. U.S.A., 1993, vol. 90.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.A. Danilova, G.N. Raldugina, Ye.A. Kunakova, A.A. Gulevich, E.N. Baranova, 2014, published in Doklady Rossiiskoi Akademii Sel’skokhozyaistvennykh Nauk, 2014, No. 5, pp. 16–21.
About this article
Cite this article
Danilova, S.A., Raldugina, G.N., Kunakova, Y.A. et al. Analysis of obtained chloroplast transformants of tobacco (Nicotiana tabacum L.) plants with marker expression cassette of aadA au gene, changing the color of leaves. Russ. Agricult. Sci. 40, 411–416 (2014). https://doi.org/10.3103/S1068367414060093
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367414060093