Abstract
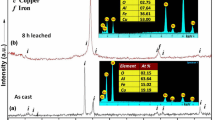
The purpose of this study is to fabricate highly dispersed powder suitable for spheroidization with further application in additive technologies. The volumetric reduction of the FeCl2–CaCl2 melt by calcium dissolved in CaCl2 produced a fine iron powder. The process included three states, notably, the preparation of melts containing FeCl2 and Ca, their mixing, and high-temperature aging at 800°C for 1 h. Upon finishing the process, the solidified fusion cake is divided into top and bottom parts. The product from the top part has a specific surface of 7.60 m2/g, and that from the bottom part has a specific surface of 5.38 m2/g. The average particle size is 157 μm for the former and 124 μm for the latter. Ultrasonic dispersing is reduced to 26 μm and 71 μm, respectively. Quantitative X-ray phase analysis shows that the main phase of powder is metallic iron (more than 97 wt %). Thus, the originality of the research is due to the application of an intense volumetric reduction of iron from chloride melts by calcium dissolved in its chloride. The uniqueness of the study is due to its product, so far as the main part of reduced iron is arranged in the melt bulk in the form of linear aggregates 40–600 μm in length and 10–50 μm in diameter, which easily destruct by ultrasonic dispersal into separate crystals with an average size of 26 μm. The results of the study show the possibility of implementing the calcium-thermal production of fine iron powder.





Similar content being viewed by others
REFERENCES
Lykov, P.A., Development of hydropneumatic aggregates of machines for the production of micropowders from liquid metals, Extend Abstract of Cand. Sci. (Eng.) Dissertation, Chelyabinsk: YuUrGU, 2014. http://tekhnosfera.com/view/447915/a?#?page=1.
Antsiferov, V.N., Bobrov, G.V., Druzhinin, L.K., Kiparisov, S.S., Kostikov, V.I., Krupin, A.V., Kudinov, V.V., Libenson, G.A., Mitin, B.S., and Roman, O.V., Poroshkovaya metallurgiya i napylennye pokrytiya (Powder Metallurgy and Spray Coatings), Moscow: Metallurgiya, 1987.
Kiparisov, S.S. and Libenson, G.A., Poroshkovaya metallurgiya (Powder Metallurgy), Moscow: Metallurgiya, 1972.
Ramakrishnan, P., Iron powder from iron scrap, Conservat. Recycl., 1983, vol. 6, no. 1, pp. 49–54. https://doi.org/10.1016/031-3658(83)90016-4
Hoeges, S., Zwiren, A., and Schade, C., Additive manufacturing using water atomized steel powders, Met. Powder Rep., 2017, vol. 72, no. 2, pp. 111–117. https://doi.org/10.1016/j.mprp.2017.01.004
Zlenko, M.A., Nagaitsev, M.V., and Dovbysh, V.M., Additivnye tekhnologii v mashinostroenii. Posobie dlya ingenerov (Additive Technologies in Mechanical Engineering. Textbook for Engineers), Moscow: NAMI, 2015, pp. 160–171.
Tsantrizos, P.G., Allaire, F., and Entezarian, M., US Patent 5707419, 1998. https://www.google.com/patents/ US5707419.
Boulos, M., Plasma power can make better powders, Metal Powder Rep., 2004, vol. 59, no. 5, pp. 16–21. https://doi.org/10.1016/S0026-0657(04)00153-5
Aleksandrov, V.G., Influence of “hot pressing” and degree of doping on the structure and properties of wares made of metallic powders, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Perm: PNIPU, 2005, http://pstu. ru/files/file/adm/dissertacii/aleksandrov/aftoreferat_ aleksandrov_vg.pdf.
Ye, Q., Zhu, H., Zhang, L., Ma, J., Zhou, L., Liu, P., Chen, J., Chen, G., and Peng, J., Preparation of reduced iron powder using combined distribution of wood-charcoal by microwave heating, J. Alloys Compd., 2014, vol. 613, pp. 102–106.https://doi.org/10.1016/j.jallcom.2014.06.016
Martin, M.I., Lopez, F.A., and Torralba, J.M., Production of sponge iron powder by reduction of rolling mill scale, Ironmak. Steelmak., 2012, vol. 39, no. 3, pp. 155–162.
Squires, A.M. and Johnson, C.A., The h-iron process, J. Met., 1957, pp. 586–590.
Brooks, J., US Patent 2762700, 1956.
Gaballah, N., Zikry, A., Khalifa, M., Farag, A., El-Hussiny, N., and Shalabi, M., Production of iron from mill scale industrial waste via hydrogen, Open J. Inorg. Non-Met. Mater., 2013, vol. 3, no. 3, pp. 23–28. https://doi.org/10.4236/ojinm.2013.33005
Bloemacher, D., Carbonyl iron powders: Its production and new developments, Met. Powder Rep., 2010, vol. 45, no. 2, pp. 117–119.https://doi.org/10.1016/S0026-0657(10)80122-5
Benchiheub, O., Mechachti, S., Serrai, S., and Khalifa, M.G., Elaboration of iron powder from mill scale, J. Mater. Envir. Sci., 2010, vol. 1, no. 4, pp. 267–276.
Despeisse, M. and Ford, S., The role of additive manufacturing in improving resource efficiency and sustainability, in Proc. Int. Conf. APMS 2015, United Kingdom: Inst. of Manufacturing, Univ. of Cambridge, 2015. http://www.apms-conference.org.
Petrick, I. and Simpson, T., 3D Printing disrupts manufacturing, Res. Technol. Manag., 2013, vol. 56, no. 6. https://doi.org/10.5437/08956308X5606193
Berman, B., 3-D Printing: The new industrial revolution, Busin. Horiz., 2012, vol. 55, pp. 155–162. https://doi.org/10.1016/j.bushor.2011.11.003
Petrovic, V., Gonzalez, J., Ferrando, O., Gordillo, J., Puchades, J., and Grinan, L., Additive layered manufacturing: Sectors of industrial application shown through case studies, Int. J. Product. Res., 2011, vol. 49, no. 4, pp. 1061–1079.https://doi.org/10.1080/00207540903479786
Gibson, I., Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, New York: Springer, 2015. https://doi.org/10.1007/978-1-4939-2113-3.
Baimakov, Yu.V. and Vetyukov, M.M., Elektroliz rasplavlennykh solei (Electrolysis of Molten Salts), Moscow: Metallurgiya, 1966.
Rodyakin, V.V., Metallurgiya kal’tsiya (Metallurgy of Calcium), Moscow: Metallurgiya, 1957.
Laptev D.A., Polyakov V.V., Babin A.V., Lebedev V.A., Reducing ability of calcium solutions in its molten chloride, in: Metallurgiya legkikh i tugoplavkikh metallov: Materialy 3-i nauchno-tekhnicheskoi konferentsii (Metallurgy of Light and Refractory Metals: Mater. 3rd Sci. and Tech. Conf.), Ekaterinburg: UrFU, 2014, pp. 169–172.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors claim that they have no conflict of interest.
Additional information
Translated by N. Korovin
About this article
Cite this article
Polyakov, V.V., Babin, A.V. & Lebedev, V.A. Volumetric Reduction of the FeCl2–CaCl2 Melt by Calcium Dissolved in Calcium Chloride. Russ. J. Non-ferrous Metals 60, 408–412 (2019). https://doi.org/10.3103/S1067821219040114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821219040114