Abstract
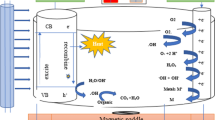
Photo-electrochemical (PEC) technology is considered a promising technique to achieve a purified ecosystem. This process is largely employed for mineralizing permanent organic compounds and recovering different metals. The aim of this article was to examine the effectiveness of photo-electrochemical treatment by utilizing a superior type of photo-catalyst (Titanium dioxide (TiO2)) as the anode (lighted by UV lamp, 320 nm) and graphite as the cathode for treating wastewater in Tehran, Iran. Wastewater samples were collected from the inlet of the Southern Tehran Wastewater Treatment Plant. NaCl (as an electrolyte) was added to the wastewater samples before the treatment to improve electrical conductivity (EC) and increase oxidants such as OH and reactive chlorine species (RCS). To optimize and study the probable effects of voltage and redox potential (Eh) on PEC efficiency, two laboratory tests were examined and lasted for 120 min. The tests showed that the best performance for all studied contaminants was at the voltage of 10 V and Eh of +138 mV (the initial Eh of the untreated wastewater). By employing a speciation study, the mechanisms of metal removal by the PEC process were investigated. The results indicated the occurrence of two types of removal, oxidation for iron (Fe), manganese (Mn) and lead (Pb), and precipitation for zinc (Zn), cadmium (Cd) and Pb. Cluster analysis also showed that the removal of contaminants was controlled by all analyzed quality parameters. These results, thus, indicated the important impacts of EC and total dissolved solids (TDS) on the mass transfer and Eh, and pH on the oxidant production and pollutants adsorption during the PEC treatment. Finally, it is recommended to continuously monitor the Eh changes for the easy assessment of wastewater quality before and after PEC treatment. This study, thus, presents an approach for assessing the PEC removal mechanisms (with excellent activity) and optimization of the ideal treating conditions (Eh and anodic voltage); also, it could serve as a database for further research to develop the PEC treatment.


Similar content being viewed by others
REFERENCES
Du, P., Zhang, L., Ma, Y., Li, X., Wang, Z., Mao, K., and Wang, X., Occurrence and fate of heavy metals in municipal wastewater in Heilongjiang province, China: A monthly reconnaissance 2015 to 2017, Water, 2020, vol. 12, no. 3, pp. 728. https://doi.org/10.3390/w12030728
Kusmierek, E., Semiconductor electrode materials applied in photoelectrocatalytic wastewater treatment, An overview, Catalysts, 2020, vol. 10, no. 4, 439. https://doi.org/10.3390/catal10040439
Mousset, E. and Dionysiou, D.D., Photoelectrochemical reactors for treatment of water and wastewater: A review, Environ. Chem. Lett., 2020, vol. 18, pp. 1301–1318. https://doi.org/10.1007/s10311-020-01014-9
Fatima, R. and Kim, J.O., Inhibiting photocatalytic electron-hole recombination by coupling MIL-125 (Ti) with chemically reduced, nitrogen-containing graphene oxide, Appl. Surf. Sci., 2021, vol. 541, 148503. https://doi.org/10.1016/j.apsusc.2020.148503
Williams, J.L., An electrolytic technique to study the mobility of inorganic constituents in soils and waste materials, PhD Thesis, Nashville: Univ. Vanderbilt, 2016.
Su, X., Kushima, A., Halliday, C., Zhou, J., Li, J., and Hatton, T.A., Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water, Nat. Commun., 2018, vol. 9, no. 1, pp. 1–9. https://doi.org/10.1038/s41467-018-07159-0
Daghrir, R., Drogui, P., Delegan, N., and El Khakani, M.A., Removal of chlortetracycline from spiked municipal wastewater using a photoelectrocatalytic process operated under sunlight irradiations, Sci. Total Environ., 2014, vol. 466, pp. 300–305. https://doi.org/10.1016/j.scitotenv.2013.07.001
Wang, D., Li, Y., Puma, G.L., Lianos, P., Wang, C., and Wang, P., Photoelectrochemical cell for simultaneous electricity generation and heavy metals recovery from wastewater, J. Hazard. Mater., 2017, vol. 323, pp. 681–689. https://doi.org/10.1016/j.jhazmat.2016.10.037
Ebraheim, G., Karbassi, A.R., and Mehrdadi, N., Chemical reducing conditions through the photo-assisted electrochemical process in the treatment of the urban rainwater, Int. J. Hum. Cap. Urban. Manage., 2021, vol. 6, no. 3, pp. 209–224. https://doi.org/10.22034/IJHCUM.2021.03.01
Karbassi, A.R., Tajziehchi, S., and Khoshgalb, H., Speciation of heavy metals in coastal water of Qeshm Island in the Persian Gulf, Global J. Environ. Sci. Manage., 2018, vol. 4, no. 1, pp. 91–98. https://doi.org/10.22034/gjesm.2018.04.01.009
Sen, B., Alp, M.T., Sonmez, F., Kocer, M.A.T., and Canpolat, O., Relationship of algae to water pollution and waste water treatment, in Water Treatment, Elshorbagy, W. and Chowdhury, R.K., Eds., London: IntechOpen, 2013, pp. 335–354.
Włodarczyk, T., Stępniewski, W., and Brzezińska, M., Dehydrogenase activity, redox potential, and emissions of carbon dioxide and nitrous oxide from cambisols under flooding conditions, Biol. Fertil. Soils, 2002, vol. 36, no. 3, pp. 200–206. https://doi.org/10.1007/s00374-002-0513-1
McMichael, S., Fernandez-Ibanez, P., and Byrne, J.A., A review of photoelectrocatalytic reactors for water and wastewater treatment, Water, 2021, vol. 13, no. 9, 1198. https://doi.org/10.3390/w13091198
Haratifar, S., Bazinet, L., Manoury, N., Britten, M., and Angers, P., Impact of redox potential electrochemical modification and storage conditions on the oxidation reaction prevention in dairy emulsion, Dairy Sci. Technol., 2011, vol. 91, no. 5, pp. 541–554. https://doi.org/10.1007/s13594-011-0025-6
Goncharuk, V.V., Bagrii, V.A., Mel’nik, L.A., Chebotareva, R.D., and Bashtan, S.Y., The use of redox potential in water treatment processes, J. Water. Chem. Technol., 2010, vol. 32, no. 1, pp. 1–9. https://doi.org/10.3103/S1063455X10010017
Papagiannis, I., Koutsikou, G., Frontistis, Z., Konstantinou, I., Avgouropoulos, G., Mantzavinos, D., and Lianos, P., Photoelectrocatalytic vs. photocatalytic degradation of organic water born pollutants, Catalysts, 2018, vol. 8, no. 10, 455. https://doi.org/10.3390/catal8100455
Etacheri, V., Di Valentin, C., Schneider, J., Bahnemann, D., and Pillai, S.C., Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments, J. Photochem. Photobiol. C: Photochem. Rev., 2015, vol. 25, pp. 1–29. https://doi.org/10.1016/j.jphotochemrev.2015.08.003
Li, X.Z. and Liu, H.S., Development of an E-H2O2/TiO2 photoelectrocatalytic oxidation system for water and wastewater treatment, Environ. Sci. Technol., 2005, vol. 39, no. 12, pp. 4614–4620. https://doi.org/10.1021/es048276k
Wahyuni, E.T., Aprilita, N.H., Hatimah, H., Wulandari, A.M., and Mudasir, M., Removal of toxic metal ions in water by photocatalytic method, Am. Chem. Sci. J., 2015, vol. 5, no. 2, pp. 194–201. https://doi.org/10.9734/ACSj/2015/13807
ALabdeh, D., Karbassi, A.R., Omidvar, B., and Sarang, A., Speciation of metals and metalloids in Anzali wetland, Iran, Int. J. Environ. Sci. Technol., 2020, vol. 17, no. 3, pp. 1411–1424. https://doi.org/10.1007/s13762-019-02471-8
De Jonge, M., Teuchies, J., Meire, P., Blust, R., and Bervoets, L., The impact of increased oxygen conditions on metal-contaminated sediments part I: Effects on redox status, sediment geochemistry and metal bioavailability, Water Res., 2012, vol. 46, no. 7, pp. 2205–2214. https://doi.org/10.1016/j.watres.2012.01.052
Funding
Not applicable. The authors did not receive support from any organization for the submitted work
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
In addition, the ethical issues, including plagiarism, informed consent, misconduct, data fabrication and/ or falsification, double publication and/or submission, and redundancy has been completely observed by the authors.
About this article
Cite this article
Gh. Ebraheim, Karbassi, A.R. & Mehrdadi, N. Employing a Photo-Electrochemical Process to Improve Wastewater Quality in Tehran, Iran. J. Water Chem. Technol. 44, 467–475 (2022). https://doi.org/10.3103/S1063455X22060042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X22060042